Heparin-binding protein (HBP) is a granule protein derived from neutrophils and located in secretory vesicles and neutrophilic granules, which is also called cationic antimicrobial protein of 37 kDa (CAP37) or leukocidin.[6] In neutrophils, HBP contributes to the regulation of endothelial permeability. HBP was considered a promising novel biomarker in infectious diseases due to the role of HBP in intravascular neutrophil activation.[7,8] In addition, HBP has been considered a predictive biomarker for the progression of organ dysfunction induced by sepsis, such as circulatory failure, respiratory failure, and acute kidney injury.[9] There is also a growing body of studies showing that a high concentration of HBP in plasma is associated with disease severity, especially mortality and organ failure.[9-12] The level of HBP in the blood of healthy people is very low, generally no more than 10 ng/ml. When an infection occurs, HBP levels change with the severity of the disease. Multiple studies have indicated that HBP is associated with various types of infection, showing elevated levels across different infections.[13] Moreover, it is considered to possess good predictive value in forecasting the occurrence, progression, and prognosis of severe infections and septic shock. In addition, recently increasing studies have illustrated that HBP has important predictive value for the prognosis of the critically ill.[12,14] Increased levels of coronary sinus HBP could serve as valuable indicators for predicting myocardial injury-related cardiogenic shock after cardiac surgery.[11] A high level of HBP in plasma upon ICU admission is connected to occurrences of respiratory and circulatory failure throughout the ICU period, which correlates with an elevated 30-day mortality rate.[15] Hence, HBP may serve as an effective biomarker for rapid clinical assessment of critical illness. However, comprehensive evaluation regarding the diagnostic efficacy of infection and prognostic ability of HBP in critically ill adult patients was warranted. Therefore, the role of HBP in the diagnosis of infection and the predictive value in critically ill adult patients were evaluated in this systematic review and meta-analysis.
Methods
Search strategy. A comprehensive systematic search was performed across PubMed, Embase, Scopus, and the Cochrane Library using a combination of Medical Subject Headings (MeSH) terms and free-text keywords. The following search terms were included: "Heparin-Binding Protein" (HBP), "Sepsis", "Diagnostic Accuracy", and "Biomarkers". Boolean operators (AND/OR) were applied for optimal search sensitivity. The detailed search strategy, including full Boolean logic and MeSH terms, is provided in SupplementaryStudy Selection. Two researchers screened the titles and abstracts of all retrieved papers by themself. The following inclusion criteria were used: (1) study participants (adults ≥ 18 years old), (2) intervention (measurements of HBP), (3) diagnosis performance in infection or prognostic performance in critically ill of HBP levels compared to other potential biomarkers, (4) The data available from the studies included were sufficient to create a 2 × 2 contingency table, which was either obtained from the original article or derived from the provided dataset or figures. Meeting abstracts, editorials, reviews, letters, case reports, and animal and cell experiments were excluded. Full-text papers were obtained if either of the researchers argued that the abstract and title were suitable. After obtaining the full papers of potentially relevant studies, two researchers independently evaluated each study's eligibility on the basis of the inclusion/exclusion criteria. Full-text papers of screened titles and abstracts in accordance with the inclusion criteria were assessed for final eligibility. The conflicting viewpoints on research eligibility were resolved through consensus negotiations or by seeking input from independent researchers (Figure 1).
Data extraction and quality assessment. Two investigators independently extracted data by applying the same standard to record the study design, publication year, country, number of study participants, participant clinical characteristics, HBP levels, biomarkers other than HBP, study outcomes, and so on. Furthermore, among the included studies, data were extracted to establish a 2 × 2 contingency table to evaluate the ability of HBP to diagnose infection in disease and predict prognosis, such as mortality and organ failure, in adult patients.
The risk of bias in the included studies was evaluated by two independent reviewers applying the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.[17] We evaluated with QUADAS-2 four potential areas of bias. These four areas include: (1) Patient selection, (2) Evaluation test, (3) Gold standard, and (4) Flow and timing. "yes", "no" or "unclear" were scored in each criterion.
Evidence synthesis. A pooled sensitivity, specificity, and AUC with the corresponding 95% CIs were calculated by applying a bivariate random-effects meta-analysis model. A hierarchical summary receiver operating characteristic (ROC) curve model was performed, and the area under the summary ROC curve with CIs was evaluated from different diagnostic and prognostic studies. In addition, the extent of heterogeneity of the included studies was quantified by the I2 statistic, and subgroup analyses were conducted to find potential sources of heterogeneity. Study design, sample size, time of study, and country were included in predefined subgroups. Potential publication bias was assessed by Deeks’ test.[18] The CIs and p-values were calculated analytically. Statistical analyses were performed by applying STATA MP17 and R software. All statistical tests were conducted with a two-tailed approach, and the statistical significance threshold was defined as p < 0.05.
Results
A flow diagram of included studies for systematic review was reported in Figure 1, which demonstrates the selection process of included studies. One thousand twenty-six studies were retrieved in our database search, and 418 studies were excluded after removing duplications. Moreover, 499 studies were further excluded after screening the titles and abstracts. Therefore, 56 studies were included after a full-text review [6,10,11,15,19-70], and 23 studies were excluded. Table 1 displays the features of the included studies. The included studies were published from 2009 to 2024. Twenty studies were performed in China, nineteen studies were conducted in Sweden, and seventeen studies were conducted in other countries. All included studies evaluated the role of HBP in diagnosing infection and predicting clinical outcomes in critically ill adult patients. Overall, the aggregate study population included a total of 11486 adult patients with infectious diseases and critically ill patients. HBP levels were used to elevate the role in adult patients. The mean age ranged between 18 and 94 years. In addition, there were 24 studies evaluating the diagnostic role of HBP, 29 studies assessing the prognostic role of HBP, and three studies evaluating both the diagnostic and predictive role of HBP. Because some researchers suggested diagnostic or prognostic accuracy separately for patients in studies, these studies were divided into two or three parts. Therefore, 38 dataset analyses were performed in the diagnosis study, and 45 dataset analyses were performed in the prognosis study.Risk of bias assessment. Problematic QUADAS items were: 1. Was a consecutive or random sample of patients enrolled? 2. Was a case-control design avoided? 3. Did the study avoid inappropriate exclusions? 4. Were the index test results interpreted without knowledge of the results of the reference standard? 5. If a threshold was used, was it pre-specified? 6. Is the reference standard likely to correctly classify the target condition? 7. Were the reference standard results interpreted without knowledge of the results of the index test? 8. Was there an appropriate interval between index test(s) and reference standard? 9. Did all patients receive a reference standard? 10. Did patients receive the same reference standard? 11. Were all patients included in the analysis?
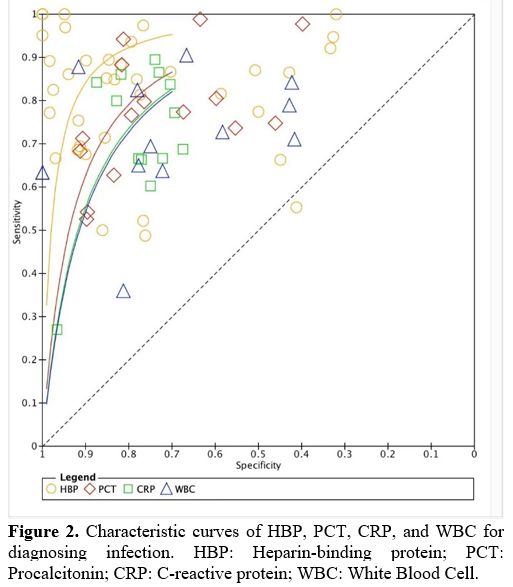
Accuracy of HBP in diagnosing infection. For the accuracy of HBP in diagnosing infection, HBP has the best discriminative power to differentiate infection from non-infection. The area under the summary ROC curve was 0.93 (95% CI, 0.91–0.95) for HBP (Figure 2), 0.85 (95% CI, 0.81–0.88) for PCT, 0.83 for CRP (95% CI, 0.79–0.86), and 0.80 (95% CI, 0.76–0.83) for WBC. Sensitivity and specificity of HBP for diagnosing infectious disease were 0.87 (95% CI, 0.82–0.91) and 0.87 (95% CI, 0.79–0.92), 0.81 (95% CI, 0.71–0.88) and 0.76 (95% CI, 0.67–0.83) for PCT, 0.74 (95% CI, 0.65–0.81) and 0.78 (95% CI, 0.72–0.83) for CRP, 0.72 (95% CI, 0.64–0.79) and 0.78 (95% CI, 0.62–0.89) for WBC.
 |
|
The value of HBP levels in predicting organ failure in critically ill adult patients. The utility of HBP levels was assessed in 12 studies to predict the future occurrence of organ failure. These studies displayed significantly higher HBP levels in adult patients who subsequently developed organ failure compared to those patients without organ failure. In our study, the area under the summary ROC curve was 0.84 (95% CI, 0.80–0.87) (Figure 3) for HBP in predicting organ failure, and sensitivity and specificity of HBP for diagnosing organ failure was 0.80 (95% CI, 0.75–0.84) and 0.72 (95% CI, 0.62–0.80). Increased plasma concentrations of HBP are correlated with an imminent risk of developing sepsis, circulatory failure, and the severity of infection in adult patients.[10] Increased HBP levels upon admission to the intensive care unit were linked to the development of severe acute kidney injury within the initial 7 days.[71]
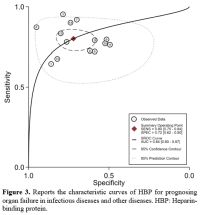 |
|
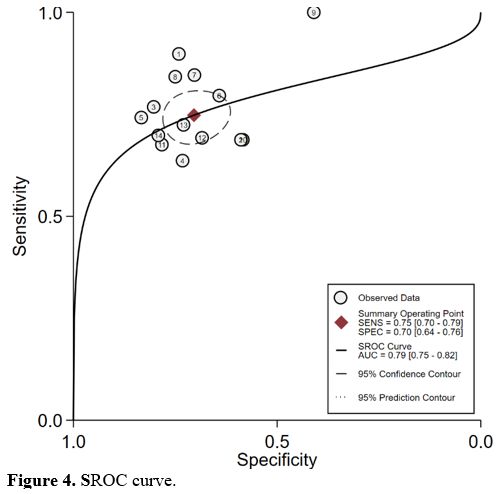
Value of HBP levels in predicting mortality in adult patients. All 14 studies evaluating the value of HBP level determination in predicting mortality in adult patients showed a significant elevation of HBP levels in non-survivors compared with that in survivors. The area under the summary ROC curve was 0.79 (95% CI, 0.75–0.82) for HBP (Figure 4). The sensitivity and specificity of HBP for diagnosing mortality were 0.75 (95% CI, 0.70–0.79) and 0.70 (95% CI, 0.64–0.76). A study showed that patients with sepsis with a decreased HBP greater than 50% in 48 hours had a greater than 90% chance of survival. However, patients with sepsis with decreased HBP in 48 hours less than 4% had a nearly 90% 30-day all-cause mortality rate.[27] Conversely, one study showed that there was no association between a single HBP level and 28-day mortality.[35]
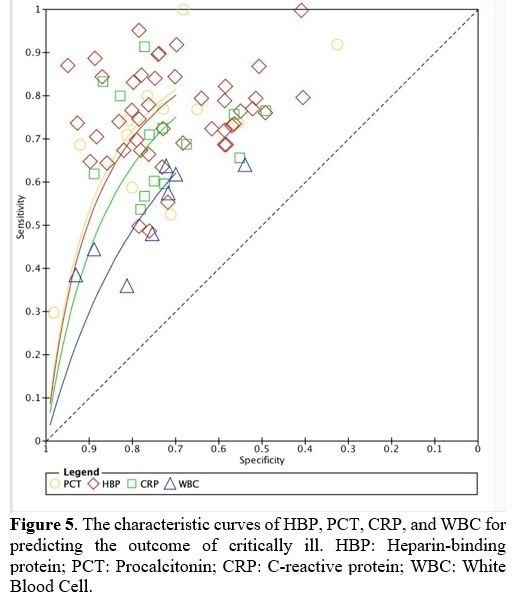
Value of HBP levels in predicting the outcome of critically ill adult patients. The association between HBP level and the outcome of critically ill adult patients was evaluated in our systematic review and meta-analysis. The results suggested that all of those with worsening conditions (including mortality and organ dysfunction) had higher HDP levels than those in stable or healthy state. In addition to mortality and organ dysfunction, the association between HBP level and the severity of diseases in adult patients has been reported in many studies. One study reported that HBP is an important biomarker in patients with ST-segment elevation typical of myocardial infarction.[32] Another study demonstrated that HBP could predict the risk of severe acute pancreatitis in advance.[38] In our present study, the area under the summary ROC curve was 0.81 (95% CI, 0.78–0.85) for HBP, and the sensitivity and specificity of HBP for prognosis in adult patients were 0.77 (95% CI, 0.74–0.80) and 0.72 (95% CI, 0.68–0.76) (Figure 5). However, one study reported that there was no association between HBP level and disease severity in patients with acute pancreatitis.[57]
Subgroup analysis. Subgroup analysis of retrospective studies (AUROC, 0.94) showed a better diagnostic accuracy of infection for HBP than that prospective (AUROC, 0.91). HBP tends to conduct better before 2020 (AUROC, 0.97) than after 2020 (including 2020) studies (AUROC, 0.90). Studies were performed on different countries that displayed similar accuracy in identifying infectious diseases. Regarding sample size, the subgroup analysis of studies from less than 200 demonstrated HBP has better discriminative power to differentiate infectious disease and non-infectious disease than that greater than 200. However, for the prognostic role of HBP in our present study, the subgroup analysis of studies from different study types, study years, and sample sizes illustrated similar results. HBP showed a better prognostic ability than Sweden and other countries in our present subgroup analysis.
Significant heterogeneity was observed in the overall analysis and subgroup analyses. However, no evidence of a threshold effect was found, which indicated that a specific cutoff point did not influence the variability in the results.
Discussion
In our systematic review and meta-analysis, we found that HBP was more accurate biomarkers for infection than CRP, PCT and WBC levels. In addition, HBP also showed good predictive ability for prognosis in critical illness, especially mortality and organ failure.For clinicians, the early identification of infections remains a challenge. Due to the emergence of antibiotic resistance, it is widely agreed that antibiotics should not be prescribed for every suspected infection. Thus, a better specific biomarker for infection would be most beneficial. HBP is a promising, innovative biomarker for predicting sepsis and sepsis-induced organ failure, which is derived from neutrophils and was first reported by Adam Linder.[10] Based on the predefined protocol, in our present systematic review and meta-analysis, which included 56 studies from several countries, we demonstrated the role of HBP in the diagnosis of infectious diseases and the development of organ failure and mortality in critically ill adult patients. The plasma HBP levels were detected in a prospective study, which included 233 subjects and was performed in 2009 to investigate human infection.[10] The study concluded that there was a strong association between high HBP levels and infection or the development of sepsis with organ dysfunction.[10] After that, HBP could be considered a useful clinical marker for infectious diseases, including sepsis. In our present study, we have summarized the diagnostic efficacy of HBP in infectious diseases. We illustrated that HBP had the best discriminative capability to distinguish infectious diseases from non-infectious diseases when compared with PCT, CRP, or WBC. As the application of biomarkers and validated clinical scoring systems becomes more prevalent in clinical practice, there has been a surge of interest in the diagnostic value of their combined utilization. Several studies have reported that HBP was combined with another biomarker to diagnose sepsis.[10,21,27,29,72] Combining HBP, PCT, and lactate may raise the diagnostic role compared to single biomarkers.[64] Therefore, combining HBP with existing biomarkers or validated clinical scoring systems could improve diagnostic accuracy for infection.
Early elevation of HBP is involved in the severity of adult patients.[35] Significantly increased HBP levels are related to a substantially elevated risk for complications in adult patients.[43] Several studies have demonstrated that most patients with aggravation of the disease have an increased HBP level ranging from 11 to 266 ng/mL.[11,56] Previous studies have also demonstrated that HBP serves as a significant predictor of mortality. One study showed that plasma HBP greater than 13.47 ng/mL was related to increased deceased patients (died), with an AUC of 0.81, a sensitivity of 0.90, and a specificity of 0.74.[19] Moreover, HBP is utilized as a diagnostic and monitoring biomarker in clinical settings to assess organ dysfunction resulting from sepsis.[43] However, HBP also has displayed a certain predictive role in organ failure in infectious diseases and other diseases.[52,56,60] Linder et al. illustrated the predictive and diagnostic role of HBP in 2009.[10] The study showed that the level of HBP increased at least 12 hours before signs of organ dysfunction at a cutoff value of 15 ng/mL in severe sepsis. HBP is increased prior to the development of organ failure in COVID-19.[46] In addition, increased plasma levels of HBP at ICU admission were independently related to multiple organ dysfunction syndrome (MODS) and early death after resuscitation from cardiac arrest.[54] In our meta-analysis, we synthesized the current evidence to display how HBP can help clinically in the monitoring of organ dysfunction in infectious diseases and other diseases. HBP plays a significant role in the pathogenesis of organ dysfunction in sepsis, specifically by causing an increase in capillary permeability.[73] This mechanism helps explain why HBP is a more effective prognostic biomarker than CRP and PCT in the detection of organ dysfunction in sepsis. In addition, some studies indicated that HBP not only predicts mortality and organ failure but also provides some indication of disease progression. One study confirmed that HBP contributed to the early identification of COPD, which illustrated that the level of HBP in the acute exacerbation group (147 ng/ml) is higher than that in the stable group (50.69 ng/ml).[26] In another study, elevated levels of coronary sinus HBP were helpful markers for predicting myocardial injury-related cardiogenic shock after cardiac surgery.[11] However, more large clinical studies are needed to establish the optimal cutoff for HBP clearance in predicting organ dysfunction and mortality.
To the best of our knowledge, the present study stands as the most comprehensive meta-analysis to date, effectively synthesizing the existing data on the role of HBP in adult patients, which includes the diagnosis of infection and the prognosis of critically ill adult patients.[72,74,75] In a previous systematic review and meta-analysis, HBP had a better diagnostic role in identifying sepsis among patients presenting with signs of systemic infection than PCT and CRP, which included 26 studies published up to 2019.[74] In our present study, we assessed the diagnostic capability of HBP for infectious diseases and evaluated its prognostic potential in predicting critically ill outcomes, which included 56 studies up to 2024. Collectively, our findings revealed that HBP illustrates not only both high sensitivity and specificity in the identification of infectious diseases but also a high prognostic role in the identification of the critically ill. In terms of clinical significance, HBP serves as a crucial diagnostic aid in detecting and ruling out infectious diseases among patients presenting with signs and symptoms of infection.[31] In addition, HBP levels are increased before the development of aggravation of disease, including organ dysfunction and mortality.[56] Moreover, this will probably lead to improved outcomes for adult patients with infectious diseases and critically ill adult patients. All the articles reported
As highlighted by Fisher et al. (2022),[76] variations influence the dynamics of circulating HBP levels in neutrophil activation and mobilization, raising concerns about its reliability in immunocompromised patients. Moreover, Chen and Ma (2024)[77] emphasize that alternative infection markers may be necessary for this patient population to enhance early detection and differentiation between infectious and non-infectious causes of fever and inflammation. Given these limitations, clinicians should be cautious when interpreting HBP levels in patients with neutropenic fever, frequently found in hematologic malignancies, and consider complementary biomarkers and clinical scoring systems to improve diagnostic accuracy.[78]
However, our present study, which also excluded neutropenic patients, has several limitations. First, variations across countries, study types, and years may contribute to heterogeneity in the pooled outcomes, potentially diminishing the precision of sensitivity and specificity measurements. Second, this study only included studies in which the language is English, and more studies in other languages are needed in future studies. Furthermore, there is limited usefulness in pooling studies comparing healthy adults and cases with studies looking at a cohort of patients with a reasonable likelihood of having the target condition.
Conclusions
While HBP has demonstrated significant utility as a biomarker for infections in critically ill patients, its reliability is reduced in individuals with hematologic malignancies, particularly those experiencing severe neutropenia. Patients with conditions such as leukemia or those undergoing intensive chemotherapy often exhibit depleted neutrophil counts, which can significantly impact the release and detection of HBP. To enhance its clinical applicability, standardized cutoff values and future multicenter studies are recommended.References
- Singer
M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M,
Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al: The Third
International Consensus Definitions for Sepsis and Septic Shock
(Sepsis-3). Jama 2016, 315(8):801-810. doi: 10.1001/jama.2016.0287. https://doi.org/10.1001/jama.2016.0287 PMid:26903338 PMCid:PMC4968574
- Cole
E, Gillespie S, Vulliamy P, Brohi K: Multiple organ dysfunction after
trauma. The British journal of surgery 2020, 107(4):402-412. doi:
10.1002/bjs.11361. https://doi.org/10.1002/bjs.11361 PMid:31691956 PMCid:PMC7078999
- Schuetz
P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt
CE, Wolff M, Chastre J et al: Effect of procalcitonin-guided antibiotic
treatment on mortality in acute respiratory infections: a patient level
meta-analysis. The Lancet Infectious diseases 2018, 18(1):95-107. doi:
10.1016/s1473-3099(17)30592-3. https://doi.org/10.1016/S1473-3099(17)30592-3 PMid:29037960
- Lambden
S, Laterre PF, Levy MM, Francois B: The SOFA score-development, utility
and challenges of accurate assessment in clinical trials. Critical care
(London, England) 2019, 23(1):374. doi: 10.1186/s13054-019-2663-7. https://doi.org/10.1186/s13054-019-2663-7 PMid:31775846 PMCid:PMC6880479
- Czajka
S, Ziębińska K, Marczenko K, Posmyk B, Szczepańska AJ, Krzych Ł J:
Validation of APACHE II, APACHE III and SAPS II scores in in-hospital
and one year mortality prediction in a mixed intensive care unit in
Poland: a cohort study. BMC anesthesiology 2020, 20(1):296. doi:
10.1186/s12871-020-01203-7. https://doi.org/10.1186/s12871-020-01203-7 PMid:33267777 PMCid:PMC7709291
- Kahn
F, Tverring J, Mellhammar L, Wetterberg N, Bläckberg A, Studahl E,
Hadorn N, Kahn R, Nueesch S, Jent P et al: Heparin-Binding Protein as a
Prognostic Biomarker of Sepsis and Disease Severity at the Emergency
Department. Shock (Augusta, Ga) 2019, 52(6):e135-e145. doi:
10.1097/shk.0000000000001332. https://doi.org/10.1097/SHK.0000000000001332 PMid:30807529
- Honore
PM, De Bels D, Barreto Gutierrez L, Redant S, Spapen HD:
Heparin-binding protein in sepsis: player! predictor! positioning?
Annals of intensive care 2019, 9(1):71. doi: 10.1186/s13613-019-0546-3.
https://doi.org/10.1186/s13613-019-0546-3 PMid:31222522 PMCid:PMC6586730
- Doherty
DE, Nakano J, Nakano K: Neutrophil-derived heparin-binding protein: a
monocyte-specific chemoattractant that induces monocyte migration into
rabbit lungs in vivo. Chest 1999, 116(1 Suppl):34s-35s. doi:
10.1378/chest.116.suppl_1.34s-a. https://doi.org/10.1378/chest.116.suppl_1.34S-a PMid:10424582
- Fisher
J, Linder A: Heparin-binding protein: a key player in the
pathophysiology of organ dysfunction in sepsis. Journal of internal
medicine 2017, 281(6):562-574. doi: 10.1111/joim.12604. https://doi.org/10.1111/joim.12604 PMid:28370601
- Linder
A, Christensson B, Herwald H, Björck L, Akesson P: Heparin-binding
protein: an early marker of circulatory failure in sepsis. Clinical
infectious diseases : an official publication of the Infectious
Diseases Society of America 2009, 49(7):1044-1050. doi: 10.1086/605563.
https://doi.org/10.1086/605563 PMid:19725785
- Pan
T, Long GF, Chen C, Zhang HT, Wang JX, Ahaskar A, Chen HB, Wang DJ:
Heparin-binding protein measurement improves the prediction of
myocardial injury-related cardiogenic shock. BMC cardiovascular
disorders 2020, 20(1):124. doi: 10.1186/s12872-020-01406-3. https://doi.org/10.1186/s12872-020-01406-3 PMid:32156261 PMCid:PMC7065315
- Linder
A, Åkesson P, Inghammar M, Treutiger CJ, Linnér A, Sundén-Cullberg J:
Elevated plasma levels of heparin-binding protein in intensive care
unit patients with severe sepsis and septic shock. Critical care
(London, England) 2012, 16(3):R90. doi: 10.1186/cc11353. https://doi.org/10.1186/cc11353 PMid:22613179 PMCid:PMC3580636
- Linder
A, Soehnlein O, Akesson P: Roles of heparin-binding protein in
bacterial infections. Journal of innate immunity 2010, 2(5):431-438.
doi: 10.1159/000314853. https://doi.org/10.1159/000314853 PMid:20505311
- Tverring
J, Nielsen N, Dankiewicz J, Linder A, Kahn F, Åkesson P: Repeated
measures of Heparin-binding protein (HBP) and procalcitonin during
septic shock: biomarker kinetics and association with cardiovascular
organ dysfunction. Intensive care medicine experimental 2020, 8(1):51.
doi: 10.1186/s40635-020-00338-8. https://doi.org/10.1186/s40635-020-00338-8 PMid:32910266 PMCid:PMC7483682
- Tydén
J, Herwald H, Sjöberg F, Johansson J: Increased Plasma Levels of
Heparin-Binding Protein on Admission to Intensive Care Are Associated
with Respiratory and Circulatory Failure. PloS one 2016,
11(3):e0152035. doi: 10.1371/journal.pone.0152035. https://doi.org/10.1371/journal.pone.0152035 PMid:27007333 PMCid:PMC4805239
- Page
MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD,
Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al: The PRISMA 2020
statement: an updated guideline for reporting systematic reviews. BMJ
(Clinical research ed) 2021, 372:n71. doi: 10.1136/bmj.n71. https://doi.org/10.1136/bmj.n71 PMid:33782057 PMCid:PMC8005924
- Whiting
PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang
MM, Sterne JA, Bossuyt PM: QUADAS-2: a revised tool for the quality
assessment of diagnostic accuracy studies. Annals of internal medicine
2011, 155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009. https://doi.org/10.7326/0003-4819-155-8-201110180-00009 PMid:22007046
- Deeks
JJ, Macaskill P, Irwig L: The performance of tests of publication bias
and other sample size effects in systematic reviews of diagnostic test
accuracy was assessed. Journal of clinical epidemiology 2005,
58(9):882-893. doi: 10.1016/j.jclinepi.2005.01.016. https://doi.org/10.1016/j.jclinepi.2005.01.016 PMid:16085191
- Acar
T, Ertekin B, Yortanlı M, Koçak S: Prognostic value of heparin-binding
protein for mortality in severe COVID-19 pneumonia. Biomarkers in
medicine 2022, 16(13):981-991. doi: 10.2217/bmm-2022-0265. https://doi.org/10.2217/bmm-2022-0265 PMid:36052658 PMCid:PMC9443788
- Akın
Şen İ, Karaşahin Ö, Şebin E, Şen C: Use of azurocidin (heparin binding
protein) and interleukin-1ß as prognostic indicators in COVID-19
patients. European Journal of Inflammation 2022, 20. doi:
10.1177/1721727x221105129. https://doi.org/10.1177/1721727X221105129 PMCid:PMC9152632
- Bergquist
M, Samuelsson L, Larsson A, Tydén J, Johansson J, Lipcsey M: TNFR1,
TNFR2, neutrophil gelatinase-associated lipocalin and heparin binding
protein in identifying sepsis and predicting outcome in an intensive
care cohort. Scientific reports 2020, 10(1):15350. doi:
10.1038/s41598-020-72003-9. https://doi.org/10.1038/s41598-020-72003-9 PMid:32948801 PMCid:PMC7501293
- Cai
R, Li H, Tao Z: Heparin-binding protein and procalcitonin in the
diagnosis of pathogens causing community-acquired pneumonia in adult
patients: a retrospective study. PeerJ 2021, 9:e11056. doi:
10.7717/peerj.11056. https://doi.org/10.7717/peerj.11056 PMid:33763308 PMCid:PMC7958890
- Chalupa
P, Beran O, Herwald H, Kaspříková N, Holub M: Evaluation of potential
biomarkers for the discrimination of bacterial and viral infections.
Infection 2011, 39(5):411-417. doi: 10.1007/s15010-011-0126-4. https://doi.org/10.1007/s15010-011-0126-4 PMid:21720792
- Dankiewicz
J, Linder A, Annborn M, Rundgren M, Friberg H: Heparin-binding protein:
an early indicator of critical illness and predictor of outcome in
cardiac arrest. Resuscitation 2013, 84(7):935-939. doi:
10.1016/j.resuscitation.2013.01.006. https://doi.org/10.1016/j.resuscitation.2013.01.006 PMid:23318914
- Dhaifalah
I, Andrys C, Drahosova M, Musilova I, Adamik Z, Kacerovsky M:
Azurocidin levels in maternal serum in the first trimester can predict
preterm prelabor rupture of membranes. The journal of maternal-fetal
& neonatal medicine: the official journal of the European
Association of Perinatal Medicine, the Federation of Asia and Oceania
Perinatal Societies, the International Society of Perinatal Obstet
2014, 27(5):511-515. doi: 10.3109/14767058.2013.820698. https://doi.org/10.3109/14767058.2013.820698 PMid:23808364
- Dong
Y, Zhou X, Zhang Y, Liu Y, Zhou X, Ren G, Li Q: Application Value of
Blood Heparin-Binding Protein in the Diagnosis of Acute Exacerbation of
Chronic Obstructive Pulmonary Disease. Contrast media & molecular
imaging 2021, 2021:3800211. doi: 10.1155/2021/3800211. https://doi.org/10.1155/2021/3800211 PMid:35024012 PMCid:PMC8716236
- Dou
QL, Liu J, Zhang W, Wang CW, Gu Y, Li N, Hu R, Hsu WT, Huang AH, Tong
HS et al: Dynamic changes in heparin-binding protein as a prognostic
biomarker for 30-day mortality in sepsis patients in the intensive care
unit. Scientific reports 2022, 12(1):10751. doi:
10.1038/s41598-022-14827-1. https://doi.org/10.1038/s41598-022-14827-1 PMid:35750778 PMCid:PMC9232494
- F.
T. Alaaraji S: Is there a Correlation between Monocyte Chemoattractant
Protein-1 with Autotaxin, Azurocidin-1, Apolipoprotein C-III and
Elastase-2 in Male Iraqi Acute Myocardial Infraction Patients?
Biomedical and Pharmacology Journal 2019, 12(04):2105-2121. doi:
10.13005/bpj/1846. https://doi.org/10.13005/bpj/1846
- Halldorsdottir
HD, Eriksson J, Persson BP, Herwald H, Lindbom L, Weitzberg E, Oldner
A: Heparin-binding protein as a biomarker of post-injury sepsis in
trauma patients. Acta anaesthesiologica Scandinavica 2018,
62(7):962-973. doi: 10.1111/aas.13107. https://doi.org/10.1111/aas.13107 PMid:29569247
- Han
X, Dou Q, Zhu Y, Ling P, Shen YH, Liu J, Zhang Z, Zhou Y, Fan M, Huang
SS et al: Heparin-binding protein-enhanced quick SOFA score improves
mortality prediction in sepsis patients. Frontiers in medicine 2022,
9:926798. doi: 10.3389/fmed.2022.926798. https://doi.org/10.3389/fmed.2022.926798 PMid:36035420 PMCid:PMC9402998
- Havelka
A, Sejersen K, Venge P, Pauksens K,Larsson A: Calprotectin, a new
biomarker for diagnosis of acute respiratory infections. Scientific
reports 2020, 10(1):4208. doi: 10.1038/s41598-020-61094-z. https://doi.org/10.1038/s41598-020-61094-z PMid:32144345 PMCid:PMC7060262
- Ipek
E, Yolcu M, Yildirim E, Altinkaynak K, Ozbek Sebin S, Kalkan K, Gulcu
O, Ermis E, Ozturk M: A Novel Marker of Inflammation: Azurocidin in
Patients with ST Segment Elevation Myocardial Infarction. International
journal of molecular sciences 2018, 19(12). doi: 10.3390/ijms19123797. https://doi.org/10.3390/ijms19123797 PMid:30501029 PMCid:PMC6321077
- Johansson
J, Brattström O, Sjöberg F, Lindbom L, Herwald H, Weitzberg E, Oldner
A: Heparin-binding protein (HBP): an early marker of respiratory
failure after trauma? Acta anaesthesiologica Scandinavica 2013,
57(5):580-586. doi: 10.1111/aas.12070. https://doi.org/10.1111/aas.12070 PMid:23320546
- Kandil
M, Khalil G, El-Attar E, Shehata G, Hassan S: Accuracy of heparin
binding protein: as a new marker in prediction of acute bacterial
meningitis. Brazilian journal of microbiology : [publication of the
Brazilian Society for Microbiology] 2018, 49 Suppl 1(Suppl 1):213-219.
doi: 10.1016/j.bjm.2018.05.007. https://doi.org/10.1016/j.bjm.2018.05.007 PMid:30166267 PMCid:PMC6328899
- Katsaros
K, Renieris G, Safarika A, Adami EM, Gkavogianni T, Giannikopoulos G,
Solomonidi N, Halvatzis S, Koutelidakis IM, Tsokos N et al: Heparin
Binding Protein for the Early Diagnosis and Prognosis of Sepsis in the
Emergency Department: The Prompt Multicenter Study. Shock (Augusta, Ga)
2022, 57(4):518-525. doi: 10.1097/shk.0000000000001900. https://doi.org/10.1097/SHK.0000000000001900 PMid:34907118
- Kjölvmark
C, Påhlman LI, Åkesson P, Linder A: Heparin-binding protein: a
diagnostic biomarker of urinary tract infection in adults. Open forum
infectious diseases 2014, 1(1):ofu004. doi: 10.1093/ofid/ofu004. https://doi.org/10.1093/ofid/ofu004 PMid:25734078 PMCid:PMC4324176
- Kjölvmark
C, Tschernij E, Öberg J, Påhlman LI, Linder A, Åkesson P:
Distinguishing asymptomatic bacteriuria from urinary tract infection in
the elderly - the use of urine levels of heparin-binding protein and
interleukin-6. Diagnostic microbiology and infectious disease 2016,
85(2):243-248. doi: 10.1016/j.diagmicrobio.2016.03.005. https://doi.org/10.1016/j.diagmicrobio.2016.03.005 PMid:27039283
- Kong
D, Lei Z, Wang Z, Yu M, Li J, Chai W, Zhao X: A novel HCP
(heparin-binding protein-C reactive protein-procalcitonin) inflammatory
composite model can predict severe acute pancreatitis. Scientific
reports 2023, 13(1):9440. doi: 10.1038/s41598-023-36552-z. https://doi.org/10.1038/s41598-023-36552-z PMid:37296194 PMCid:PMC10256784
- Kong
Y, Ye Y, Ma J, Shi G: Accuracy of heparin-binding protein for the
diagnosis of nosocomial meningitis and ventriculitis. Critical care
(London, England) 2022, 26(1):56. doi: 10.1186/s13054-022-03929-x. https://doi.org/10.1186/s13054-022-03929-x PMid:35260175 PMCid:PMC8903701
- Leppilahti
JM, Hernández-Ríos PA, Gamonal JA, Tervahartiala T,
Brignardello-Petersen R, Mantyla P, Sorsa T, Hernández M: Matrix
metalloproteinases and myeloperoxidase in gingival crevicular fluid
provide site-specific diagnostic value for chronic periodontitis.
Journal of clinical periodontology 2014, 41(4):348-356. doi:
10.1111/jcpe.12223. https://doi.org/10.1111/jcpe.12223 PMid:24382144
- Lin
Q, Shen J, Shen L, Zhang Z, Fu F: Increased plasma levels of
heparin-binding protein in patients with acute respiratory distress
syndrome. Critical care (London, England) 2013, 17(4):R155. doi:
10.1186/cc12834. https://doi.org/10.1186/cc12834 PMid:23883488 PMCid:PMC4056599
- Linder
A, Akesson P, Brink M, Studahl M, Björck L, Christensson B:
Heparin-binding protein: a diagnostic marker of acute bacterial
meningitis. Critical care medicine 2011, 39(4):812-817. doi:
10.1097/CCM.0b013e318206c396. https://doi.org/10.1097/CCM.0b013e318206c396 PMid:21200320
- Linder
A, Arnold R, Boyd JH, Zindovic M, Zindovic I, Lange A, Paulsson M,
Nyberg P, Russell JA, Pritchard D et al: Heparin-Binding Protein
Measurement Improves the Prediction of Severe Infection With Organ
Dysfunction in the Emergency Department. Critical care medicine 2015,
43(11):2378-2386. doi: 10.1097/ccm.0000000000001265. https://doi.org/10.1097/CCM.0000000000001265 PMid:26468696 PMCid:PMC4603368
- Ma
H, Liu H, Wu C, Huang L: Diagnostic Value of Serum Heparin Binding
Protein, Blood Lactic Acid Combined with hs-CRP in Sepsis and Its
Relationship with Prognosis. Evidence-based complementary and
alternative medicine : eCAM 2021, 2021:5023733. doi:
10.1155/2021/5023733. https://doi.org/10.1155/2021/5023733 PMid:34795784 PMCid:PMC8594982
- Ma
J, Lu Q, Tu S, Miao X, Zhao J: A diagnostic test: combined detection of
heparin-binding protein, procalcitonin, and C-reactive protein to
improve the diagnostic accuracy of bacterial respiratory tract
infections. Journal of thoracic disease 2022, 14(3):721-728. doi:
10.21037/jtd-22-260. https://doi.org/10.21037/jtd-22-260 PMid:35399251 PMCid:PMC8987818
- Mellhammar
L, Thelaus L, Elén S, Fisher J, Linder A: Heparin binding protein in
severe COVID-19-A prospective observational cohort study. PloS one
2021, 16(4):e0249570. doi: 10.1371/journal.pone.0249570. https://doi.org/10.1371/journal.pone.0249570 PMid:33822821 PMCid:PMC8023466
- Meng
Y, Zhang L, Huang M, Sun G: Blood heparin-binding protein and
neutrophil-to-lymphocyte ratio as indicators of the severity and
prognosis of community-acquired pneumonia. Respiratory medicine 2023,
208:107144. doi: 10.1016/j.rmed.2023.107144. https://doi.org/10.1016/j.rmed.2023.107144 PMid:36736745
- Nalmpantis
D, Gatou A, Fragkioudakis I, Margariti A, Skoura L, Sakellari D:
Azurocidin in gingival crevicular fluid as a potential biomarker of
chronic periodontitis. Journal of periodontal research 2020,
55(2):209-214. doi: 10.1111/jre.12703. https://doi.org/10.1111/jre.12703 PMid:31608993
- Namiduru
E NM, Karaoğlan I, Erbağci E: Heparin Binding Protein in Early
Differential Diagnosis of Bacterial Meningitis. Indian Journal of
Clinical Biochemistry 2022. doi: 10.1007/s12291-022-01066-4. https://doi.org/10.1007/s12291-022-01066-4 PMid:38223001 PMCid:PMC10784236
- Obreja
M, Miftode EG, Stoleriu I, Constantinescu D, Vâță A, Leca D, Cianga CM,
Dorneanu OS, Pavel-Tanasa M, Cianga P: Heparin-Binding Protein (HBP),
Neutrophil Gelatinase-Associated Lipocalin (NGAL) and S100
Calcium-Binding Protein B (S100B) Can Confirm Bacterial Meningitis and
Inform Adequate Antibiotic Treatment. Antibiotics (Basel, Switzerland)
2022, 11(6). doi: 10.3390/antibiotics11060824. https://doi.org/10.3390/antibiotics11060824 PMid:35740230 PMCid:PMC9220165
- Olinder
J, Börjesson A, Norrman J, West T, Carlström J, Gustafsson A, Annborn
M, Herwald H, Rydén C: Hepcidin discriminates sepsis from other
critical illness at admission to intensive care. Scientific reports
2022, 12(1):14857. doi: 10.1038/s41598-022-18826-0. https://doi.org/10.1038/s41598-022-18826-0 PMid:36050405 PMCid:PMC9434539
- Pajenda
S, Figurek A, Wagner L, Gerges D, Schmidt A, Herkner H, Winnicki W:
Heparin-binding protein as a novel biomarker for sepsis-related acute
kidney injury. PeerJ 2020, 8:e10122. doi: 10.7717/peerj.10122. https://doi.org/10.7717/peerj.10122 PMid:33088624 PMCid:PMC7568480
- Paulsson
M, Thelaus L, Riesbeck K, Qvarfordt I, Smith ME, Lindén A, Linder A:
Heparin-binding protein in lower airway samples as a biomarker for
pneumonia. Respiratory research 2021, 22(1):174. doi:
10.1186/s12931-021-01764-2. https://doi.org/10.1186/s12931-021-01764-2 PMid:34103069 PMCid:PMC8185500
- Ristagno
G, Masson S, Tiainen M, Bendel S, Bernasconi R, Varpula T, Milani V,
Vaahersalo J, Magnoli M, Spanuth E et al: Elevated plasma
heparin-binding protein is associated with early death after
resuscitation from cardiac arrest. Critical care (London, England)
2016, 20(1):251. doi: 10.1186/s13054-016-1412-4. https://doi.org/10.1186/s13054-016-1412-4 PMid:27497949 PMCid:PMC4976065
- Saridaki
M, Metallidis S, Grigoropoulou S, Vrentzos E, Lada M, Argyraki K,
Tsachouridou O, Georgiadou A, Vasishta A, Giamarellos-Bourboulis EJ:
Integration of heparin-binding protein and interleukin-6 in the early
prediction of respiratory failure and mortality in pneumonia by
SARS-CoV-2 (COVID-19). European journal of clinical microbiology &
infectious diseases : official publication of the European Society of
Clinical Microbiology 2021, 40(7):1405-1412. doi:
10.1007/s10096-020-04145-7. https://doi.org/10.1007/s10096-020-04145-7 PMid:33515095 PMCid:PMC7846268
- Shu
W, Wan J, Yang X, Chen J, Yang Q, Liu F, Xia L: Heparin-Binding Protein
Levels at Admission and Within 24 h Are Associated with Persistent
Organ Failure in Acute Pancreatitis. Digestive diseases and sciences
2021, 66(10):3597-3603. doi: 10.1007/s10620-020-06660-1. https://doi.org/10.1007/s10620-020-06660-1 PMid:33094452
- Sjöbeck
M, Sternby H, Herwald H, Thorlacius H, Regnér S: Heparin-binding
protein is significantly increased in acute pancreatitis. BMC
gastroenterology 2021, 21(1):337. doi: 10.1186/s12876-021-01910-6. https://doi.org/10.1186/s12876-021-01910-6 PMid:34454419 PMCid:PMC8403433
- Stjärne
Aspelund A, Hammarström H, Inghammar M, Larsson H, Hansson L,
Christensson B, Påhlman LI: Heparin-binding protein, lysozyme, and
inflammatory cytokines in bronchoalveolar lavage fluid as diagnostic
tools for pulmonary infection in lung transplanted patients. American
journal of transplantation : official journal of the American Society
of Transplantation and the American Society of Transplant Surgeons
2018, 18(2):444-452. doi: 10.1111/ajt.14458. https://doi.org/10.1111/ajt.14458 PMid:28787761 PMCid:PMC5813223
- Sun
JK, Shen X, Sun XP, Wang X, Zhang WH, Shi QK, Mu XW: Heparin-binding
protein as a biomarker of gastrointestinal dysfunction in critically
ill patients: a retrospective cross-sectional study in China. BMJ open
2020, 10(7):e036396. doi: 10.1136/bmjopen-2019-036396. https://doi.org/10.1136/bmjopen-2019-036396 PMid:32624474 PMCid:PMC7337894
- Tang
J, Yuan H, Wu YL, Fu S, Pan XY: The Predictive Value of Heparin-Binding
Protein and D-Dimer in Patients with Sepsis. International journal of
general medicine 2023, 16:2295-2303. doi: 10.2147/ijgm.S409328. https://doi.org/10.2147/IJGM.S409328 PMid:37304904 PMCid:PMC10257474
- Temiz
A AK, Albayrak Y, Özdemir Y, Şebin S, anrıkulu C: Prognostic importance
of plasma heparin binding protein in the diagnosis of acute
appendicitis. Annals of Clinical and Analytical Medicine 2019, 10(04).
doi: 10.4328/acam.6004. https://doi.org/10.4328/ACAM.6004
- Tian
R, Chen X, Yang C, Teng J, Qu H, Liu HL: Serum Heparin-Binding Protein
as a Potential Biomarker to Distinguish Adult-Onset Still's Disease
From Sepsis. Frontiers in immunology 2021, 12:654811. doi:
10.3389/fimmu.2021.654811. https://doi.org/10.3389/fimmu.2021.654811 PMid:33868298 PMCid:PMC8044511
- Tverring
J, Vaara ST, Fisher J, Poukkanen M, Pettilä V, Linder A:
Heparin-binding protein (HBP) improves prediction of sepsis-related
acute kidney injury. Annals of intensive care 2017, 7(1):105. doi:
10.1186/s13613-017-0330-1. https://doi.org/10.1186/s13613-017-0330-1 PMid:29047023 PMCid:PMC5647316
- Wang
Z, Chang B, Zhang Y, Chen J, Xie F, Xiang Y, Liu T, Li Y: Clinical
value of serum sTREM-1 and HBP levels in combination with traditional
inflammatory markers in diagnosing hospital-acquired pneumonia in
elderly. BMC infectious diseases 2022, 22(1):773. doi:
10.1186/s12879-022-07758-9. https://doi.org/10.1186/s12879-022-07758-9 PMid:36195852 PMCid:PMC9531631
- Widén
J, Cederberg D, Linder A, Westman G: Heparin-binding protein as a
marker of ventriculostomy related infection and central nervous system
inflammation in neuro-intensive care. Clinical neurology and
neurosurgery 2023, 229:107752. doi: 10.1016/j.clineuro.2023.107752. https://doi.org/10.1016/j.clineuro.2023.107752 PMid:37156040
- Wu
KA, Wu CC, Liu YC, Hsueh PC, Chin CY, Wang CL, Chu CM, Shih LJ, Yang
CY: Combined serum biomarkers in the noninvasive diagnosis of
complicated parapneumonic effusions and empyema. BMC pulmonary medicine
2019, 19(1):108. doi: 10.1186/s12890-019-0877-8. https://doi.org/10.1186/s12890-019-0877-8 PMid:31215423 PMCid:PMC6582530
- Ying Li,Ke Feng WC, Jing Qiao, Meng Zhao: HIGH LEVELS OF CRP, PCT AND HBP ARE COLLRELATED WITH PNEUMONIA SECONDARY SEPSIS. Acta Medica Mediterranea 2020, 36:2173-2177. doi: 10.19193/0393-6384_2020_4_338.
- Zhang S ZY, Shi B, Chen X, Zhang H, Li C, Jun Zhang, Hong P, Gao S: Evaluation of heparin-binding protein and or procalcitonin levels in the diagnosis of bacterial intracranial infection using receiver operating characteristic (ROC) curve value. Int J Clin Exp Med 2019, 12(6):7778-7782.
- Zhong
Y, Yu Z, Wang L, Yang X: Combined detection of procalcitonin,
heparin-binding protein, and interleukin-6 is a promising assay to
diagnose and predict acute pancreatitis. Journal of clinical laboratory
analysis 2021, 35(8):e23869. doi: 10.1002/jcla.23869. https://doi.org/10.1002/jcla.23869 PMid:34151489 PMCid:PMC8373338
- Zhou
Y, Liu Z, Huang J, Li G, Li F, Cheng Y, Xie X, Zhang J: Usefulness of
the heparin-binding protein level to diagnose sepsis and septic shock
according to Sepsis-3 compared with procalcitonin and C reactive
protein: a prospective cohort study in China. BMJ open 2019,
9(4):e026527. doi: 10.1136/bmjopen-2018-026527. https://doi.org/10.1136/bmjopen-2018-026527 PMid:31015272 PMCid:PMC6502053
- Tydén J, Herwald H, Hultin M, Walldén J, Johansson J: Heparin-binding protein as a biomarker of acute kidney injury in critical illness. Acta anaesthesiologica Scandinavica 2017, 61(7):797-803. doi: 10.1111/aas.12913. https://doi.org/10.1111/aas.12913 PMid:28585315
- Chen S, Zhang C, Hong G, Wang Q, Lian M: Meta-analysis of the diagnostic efficacy of heparin binding protein in adult sepsis. Zhonghua wei zhong bing ji jiu yi xue 2019, 31(11):1330-1334. doi: 10.3760/cma.j.issn.2095-4352.2019.11.004.
- Tapper
H, Karlsson A, Mörgelin M, Flodgaard H, Herwald H: Secretion of
heparin-binding protein from human neutrophils is determined by its
localization in azurophilic granules and secretory vesicles. Blood
2002, 99(5):1785-1793. doi: 10.1182/blood.v99.5.1785. https://doi.org/10.1182/blood.V99.5.1785 PMid:11861296
- Wu
YL, Yo CH, Hsu WT, Qian F, Wu BS, Dou QL, Lee CC: Accuracy of
Heparin-Binding Protein in Diagnosing Sepsis: A Systematic Review and
Meta-Analysis. Critical care medicine 2021, 49(1):e80-e90. doi:
10.1097/ccm.0000000000004738. https://doi.org/10.1097/CCM.0000000000004738 PMid:33196528
- Chen M, Yuan J, Yang Z, Cai G: Value of heparin-binding protein in diagnosis of sepsis in adult patients: a Meta-analysis. Zhonghua wei zhong bing ji jiu yi xue 2019, 31(10):1224-1230. doi: 10.3760/cma.j.issn.2095-4352.2019.10.009.
- Fisher J, Kahn F, Wiebe E, et al. The Dynamics of Circulating Heparin-Binding Protein: Implications for Its Use as a Biomarker. J Innate Immun. 2022;14(5):447-460. doi:10.1159/000521064 https://doi.org/10.1159/000521064 PMid:34965528 PMCid:PMC948591
- Chen Y, Ma T. Hematologic cancers and infections: how to detect infections in advance and determine the type? Front Cell Infect Microbiol. 2024 Nov 4;14:1476543. doi: 10.3389/fcimb.2024.1476543. https://doi.org/10.3389/fcimb.2024.1476543 PMid:39559703 PMCid:PMC11570547
- Kostic I., Gurrieri C., Piva E., Semenzato G., Plebani M., Caputo I., Vianello F.Comparison of presepsin, procalcitonin, interleukin-8 and C-reactive protein in predicting bacteraemia in febrile neutropenic adult patients with haematological malignancies. Mediterr J Hematol Infect Dis 2019, 11(1): e2019047, https://doi.org/10.4084/mjhid.2019.047 PMid:31528313 PMCid:PMC6736337