Thalassemia encompasses a broad spectrum of disorders, including compound heterozygous states that combine hemoglobin variants with thalassemia mutations, for instance, sickle cell thalassemia.[3] Similarly, Hb E beta thalassemia - common in Southeast Asia - results from the co-inheritance of Hb E and beta-thalassemia genes, with disease severity ranging from asymptomatic to transfusion-dependent.[4] Another variant, Hb H disease, a form of alpha-thalassemia caused by the deletion of three alpha-globin genes, leads to hemolytic anemia due to unstable Hb H tetramers.[5] Clinically, thalassemia is divided into two types depending on the need for regular blood transfusions: non-transfusion-dependent thalassemia (NTDT) and transfusion-dependent thalassemia (TDT).[6] TDT requires chronic blood transfusions to manage persistent anemia caused by the ineffective production of red blood cells.[7] While transfusions are essential for sustaining life and for normal growth and development, their repeated application introduces a range of complex complications over time, making long-term management increasingly challenging.[8]
Iron overload is a serious complication arising from frequent transfusions and intestinal absorption, resulting in toxic iron accumulation in critical organs.[9] Iron chelation therapy is commonly used to counteract excess iron in the body. In contrast, chelation therapy can be effective in managing iron overload; it is not without its risks, including renal dysfunction, hepatic toxicity, and gastrointestinal disturbances, all of which can complicate the clinical picture.[10] Therefore, understanding the full spectrum of complications from transfusions, iron chelation therapy, and associated interventions is essential for optimizing the care for thalassemia patients.[7]
In addition to iron overload, infections are the second leading cause of death in TDT patients, representing a primary concern in patient management.[11] The risk factors for infections in these patients can be attributed to the disease, such as anemia and dysfunction of the reticuloendothelial system, and treatment-related factors, such as iron chelation, transfusion-related infections, and splenectomy.[12]
In thalassemia, defective red blood cells are prematurely destroyed by the spleen, leading to splenomegaly, where the spleen becomes enlarged and overactive.[13] This excessive removal of red blood cells contributes to ongoing anemia, thrombocytopenia، and even leukopenia. In many cases, splenectomy is performed to address this issue both in TDT and NTDT. Splenectomy can lead to an increase in hemoglobin levels by 1-2 g/dl, helping to alleviate symptoms related to anemia and splenomegaly.[13] However, while splenectomy can decrease transfusion dependency, it also increases the risk of severe infections, as the spleen plays a vital role in the body's defense against infections, particularly those caused by encapsulated bacteria.[14] Several preventive approaches are critical to mitigate the increased risk of infections following splenectomy, including vaccination, antibiotic prophylaxis, and patient education on the importance of infection prevention.[15]
Thalassemia is highly prevalent in Saudi Arabia, significantly causing a burden on tertiary care centers,[16] and that highlights the need for optimized management protocols that address the cumulative complications arising from transfusion dependency, iron chelation, and splenectomy.[17] However, it has been noted that there is a significant lack of comprehensive studies on the incidence and risk factors of infections in thalassemia patients in Saudi Arabia.
Identifying risk factors is essential for enhancing patient monitoring, treatment, and overall health outcomes. By understanding these factors, healthcare providers can design more targeted interventions, providing better care for individuals at greater risk. This approach ultimately helps improve the overall health and well-being of this vulnerable population.
Methods
Participants and Study Design. This retrospective study utilized hospital medical records from a tertiary care center. Patients with Thalassemia syndrome, including those with TDT and NTDT, were included in the analysis from 2007 to 2022. All data was anonymized to ensure confidentiality and securely maintained in the office of the corresponding author, in compliance with the Declaration of Helsinki. The study was approved by Biomedical Ethics at the Faculty of Medicine and KAUH (No. 511-20). The study followed the STROBE guidelines for reporting data.Initially, a total of 986 patients with hemoglobinopathy were screened. Then, 387 medical records of patients with thalassemia syndromes were reviewed, and patients with thalassemia minor and those with insufficient data were excluded, resulting in a final sample of 303 patients. Among these, 244 patients were above the age of 14, while 59 were below the age of 14 at the time of data collection.
Participants' Clinical and Laboratory Information. Data collected included patient demographics such as age, gender, nationality, and BMI. Transfusion status, hemoglobin electrophoresis, vaccination history (patients were considered fully vaccinated if they received all vaccines against encapsulated organisms (Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis), splenectomy status, and other comorbidities were recorded. We collected all culture types, noting the cultures' date, site, and results. Furthermore, we obtained data on chelation status, mean ferritin values, and the most recent lab results for albumin, bilirubin, Hemoglobin A1c, Hepatitis B, Hepatitis C, and HIV status. Lastly, results from the last imaging studies, including abdominal ultrasound, echocardiogram, and MRI for iron overload assessment, were also reviewed.
Data Analysis. Initially, the characteristics of study patients were described using the mean for continuous variables and proportions for categorical variables. Group differences were compared between patients with a positive culture and those with no positive culture using chi-square or Fisher's exact test as appropriate for categorical variables and a two-sample t-test for numerical variables.
Following the above descriptive analysis of the study sample and the different variables, a stepwise approach was used to construct a multivariate logistic regression model to evaluate the effect of chelation therapy, vaccination status, splenectomy status, ferritin levels, and type of thalassemia on positivity, adjusting for age and gender. Cases with incomplete values for the variables included in the final model were excluded from the analysis. The odds ratio was calculated, and 95% confidence intervals (CIs) were reported. A p-value of less than 0.05 was considered statistically significant. Moreover, an ANOVA test was conducted to compare ferritin values in patients who had one, two or three positive cultures. All statistical analysis was performed using Stata (ver. 18.0).
Results
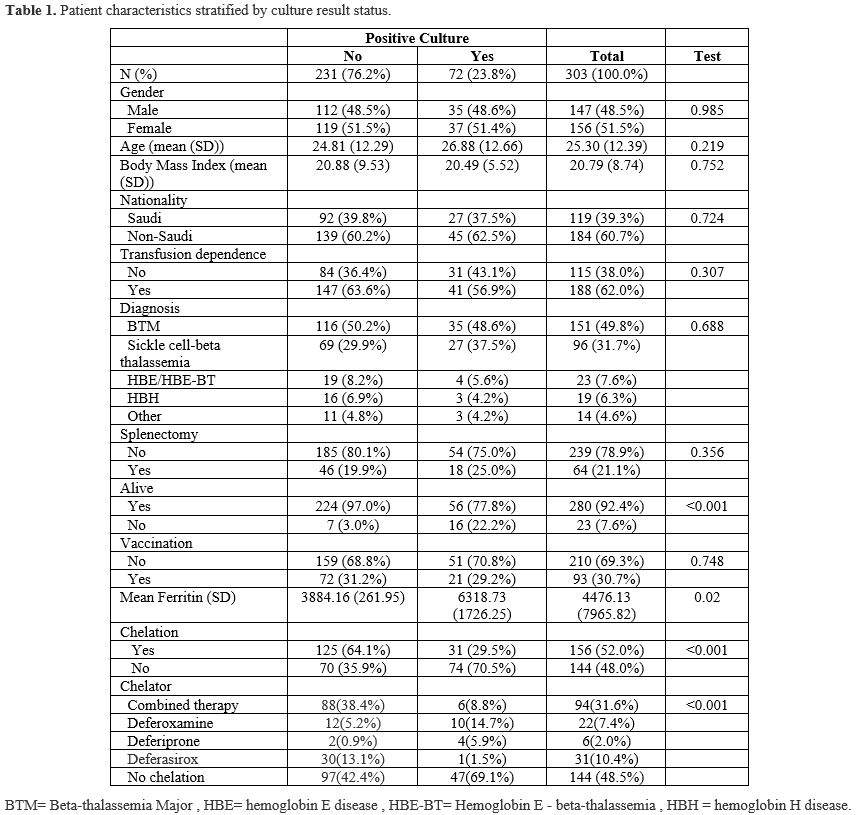
Three hundred three patients were included in this retrospective review; of those, 244 patients were above 14 years old (the cutoff for admission into the adult ward in our hospital), and 59 pediatric patients. 51.49% were females and 48.51% were males. One hundred eighty-eight patients were transfusion-dependent, while the remaining 115 patients were NTDT. Genetically, 151 were beta-thalassemia major patients, 96 were sickle beta-thalassemia, 23 had hemoglobin E or hemoglobin E beta-thalassemia, and 16 had Hemoglobin H disease (HBH). Most patients (61%) were non-Saudi nationals, while 39% were Saudis. Table 1 summarizes the study population separated into two groups based on the outcome variable of having any positive culture.Seventy-two patients had a total of 133 positive cultures from different sites, including blood, urine, respiratory tract, and other sites. Among these patients, 47 had one positive culture each, 15 had two positive cultures, three patients had three cultures, two had four positive cultures, two had six, and one patient had seven, nine, and 11 positive cultures each. Twelve of these infections resulted in ICU admission. A summary of the organisms detected in cultures is presented in Table 3, which includes 30 bloodstream infections, 63 urinary tract infections, 16 respiratory tract infections, and 24 other infections at other sites. Escherichia coli was the most prevalent pathogen causing bloodstream infection in our patients (27%), and the most common pathogen causing urinary tract infection (33%). There was no statistically significant difference between the positive culture and negative culture groups regarding gender, BMI, transfusion dependency, history of splenectomy, history of vaccination, or blood group. Patients with positive cultures were older and more likely to have iron overload and not be on chelation therapy. Moreover, mortality rates were higher among patients with positive cultures. A total of 23 patients passed away. Among the 19 patients with a clearly documented cause of death, sepsis was identified as the cause in 12 cases (63%). Mortality rates were notably higher among patients with bloodstream infections compared to those with infections at other sites (33% vs. 18%).
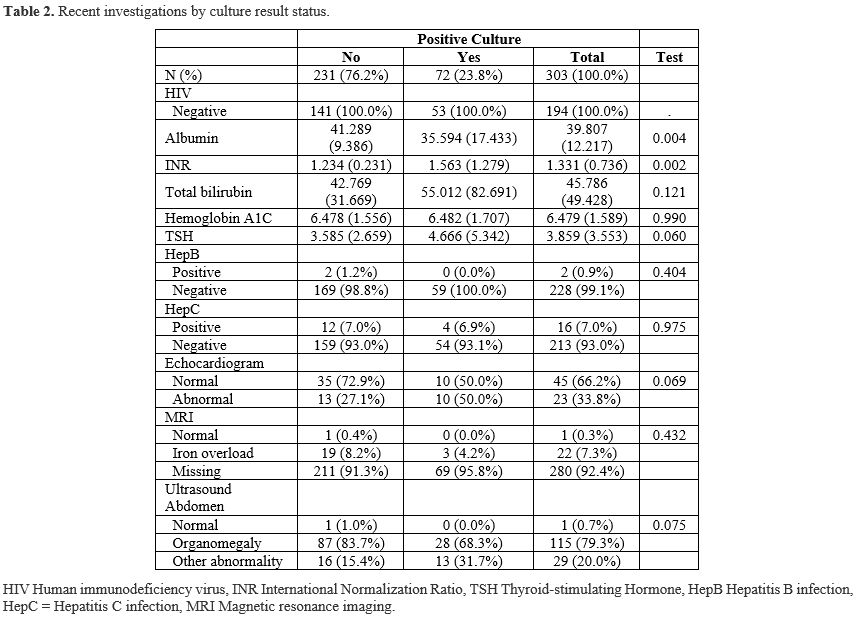 |
Table 2. Recent investigations by culture result status. |
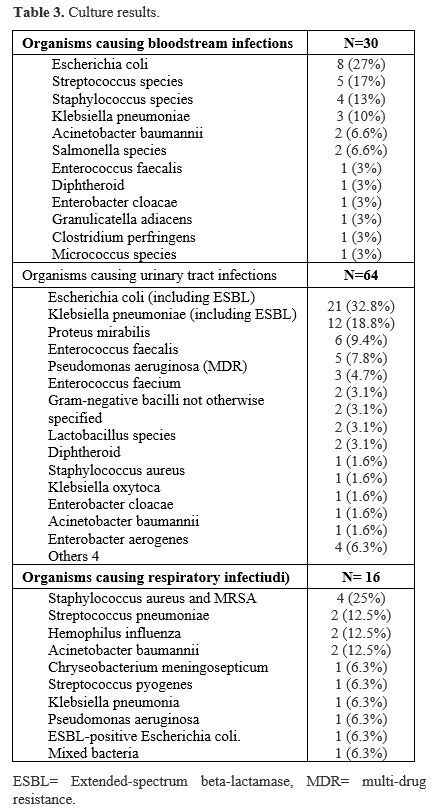 |
Table 3. Culture results. |
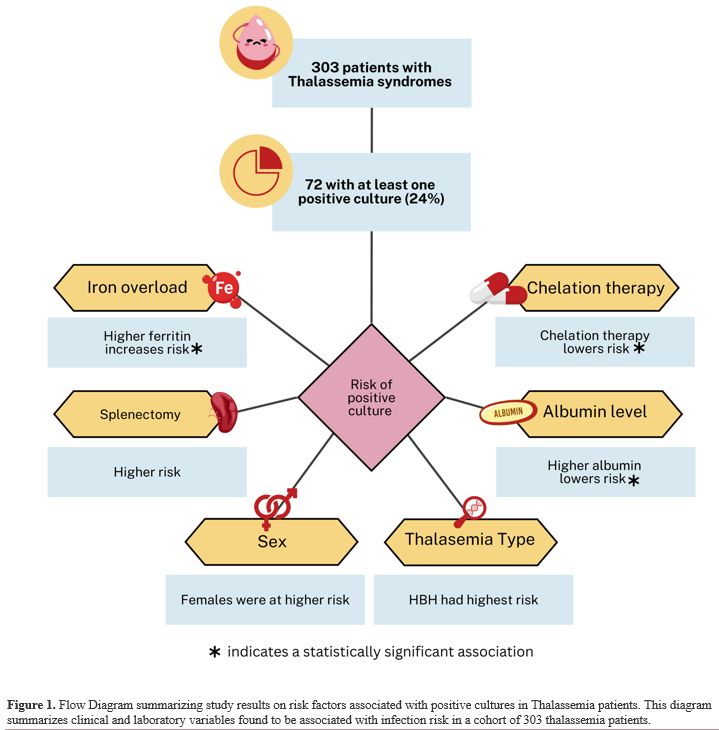
A multivariate logistic regression was conducted to evaluate the potentially relevant risk factors further. The analysis revealed that higher ferritin levels were associated with an increased likelihood of positive cultures; the odds ratio indicates that for every one-unit increase in ferritin, the odds of a positive culture increase very slightly (by 0.01%) (odds ratio: 1.0001, p = 0.00). Chelation therapy was significantly protective, with patients on chelation having substantially lower odds of infection (OR 0.18, 95% CI: 0.06–0.48, p < 0.001). Female patients had higher odds of developing an infection, although this was not statistically significant (OR 1.55, p = 0.27). Similarly, patients with a history of splenectomy showed increased odds of infection, but this association also did not reach statistical significance (OR 2.28, p = 0.12). Interestingly, when analyzing the chelators separately, it seems that patients on combined chelation therapy had a statistically significant protective effect against having a positive culture (OR 0.02, P=0.01). Moreover, a higher Albumin level was associated with marginally lower odds of developing an infection (OR 0.92, p = 0.00). Interestingly, patients with HBH seem to have the highest odds of a culture-positive result compared to other types of thalassemia (OR 1.12, P=0.89). For complete details of the logistic regression analysis, please refer to Table 4. For a summary of the study's results, refer to Figure 1.
We noted low utilization of MRI as the gold standard test for assessing iron overload. This may be due to several factors, including limited availability of imaging services and a lack of patient interest in pursuing the procedure. Among patients who did undergo MRI, 95.6% had confirmed iron overload based on the imaging findings.
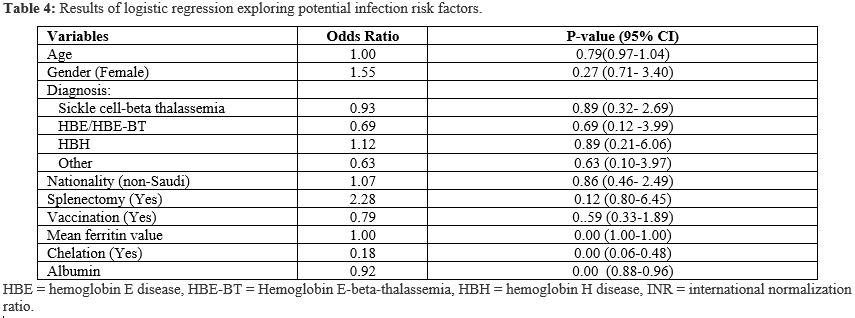 |
Table 4. Results of logistic regression exploring potential infection risk factors. |
Discussion
In this study, we investigate the complex relationship between infection risk, iron overload, and chelation in thalassemia patients. By examining demographic factors, laboratory findings, and clinical complications, we explored the factors influencing infection risk in TDT, NTDT, and sickle thalassemia patients. Thalassemia patients with infections in this study were older and more likely to have iron overload and not to be on chelation therapy. Moreover, mortality rates were higher among patients with positive cultures. These findings can inform the creation of successful management and treatment approaches.The current study showed that 23.8% of patients had positive cultures from several locations, including blood, urine, and respiratory tracts. A similar study, conducted in 2020, found that 65.8% of infected thalassemia patients had positive cultures.[18] A study from Pakistan indicated that 25.3% of 1253 individuals who were repeatedly transfused were infected with Transfusion Transmitted Infections (TTIs).[19] Other studies suggested that the incidence of infection in thalassemia major patients ranges from 22.5% to 66%.[20,21] Furthermore, a 2015 study in Thailand on 211 thalassemia patients found serious bacterial infections in 5.2% of them.[22]
Our findings demonstrated that elevated ferritin levels - a key indicator of iron overload - were significantly associated with an increased risk of infection. Excess iron in the body creates a favorable environment for bacterial growth, thereby heightening susceptibility to infections.[23] This observation aligns with earlier research indicating a link between iron overload and an increased risk of infection, particularly before starting iron-chelating medication.[24] Teawtrakul et al. found that serum ferritin levels more than 1000 ng/ml were a clinical risk factor for severe bacterial infection in NTDT patients.[22] However, ferritin is an acute-phase reactant, and its elevation during infections may not solely reflect iron overload.[25] To address this, we calculated mean ferritin levels. This approach aimed to provide a more stable estimate of baseline iron status, though it may not fully eliminate the influence of acute-phase responses.
Clinical research has yet to clearly demonstrate the significance of iron load in susceptibility to infection. However, iron overload increases the pathogenicity of a variety of microorganisms. Many pathogens, including Klebsiella species, Escherichia coli, Streptococcus pneumonia, Pseudomonas aeruginosa, Legionella pneumophila, and Listeria monocytogenes, have been demonstrated to be more virulent in the presence of excess iron.[26] Studies on the consequences of chronic iron overload on the immune system have shown that it is related to impaired neutrophil and macrophage chemotaxis, phagocytosis, and decreased bactericidal activity, all of which contribute to diminished immunological function.[27] Furthermore, chronic iron overload has been shown to impede bacterial killing of human monocytic cell line (THP-1) derived macrophages by inhibiting lysosomal acidification.[28] Maintaining iron homeostasis is essential for oxygen transport and numerous cellular functions, including DNA synthesis, mitochondrial activity, and the activation and proliferation of CD4+ T cells.[29]
In contrast, iron overload can trigger ferroptosis - a form of iron-dependent cell death - leading to the dysfunction and loss of T cells.[30] One recent study demonstrated that L-Citrulline supplementation protects against iron overload in the mouse thymus by inhibiting ferritinophagy and ferroptosis. The authors concluded that L-Citrulline may act as an iron chelator with antioxidant and anti-inflammatory properties, offering a potential therapeutic strategy to mitigate thymic oxidative damage and immune dysfunction caused by iron overload.[31] Although not part of the study's primary objectives, the findings provide some light on the suboptimal follow-up on iron overload in many of the study patients.
The relationship between chelation therapy and the risk of infection is complex to explore, given so many potential confounding factors, including patient compliance, route of administration and specific agent type, concomitant medication, comorbidities, and other environmental factors. Overall, chelation therapy is well established to increase the survival and quality of life of thalassemia patients.[10] Iron chelation therapy has been linked to an increased risk of some serious bacterial infections.[12] Deferoxamine, for example, has been found as a siderophore for specific bacteria, including Mucormycosis and Yersinia enterocolitica, which increases virulence and worsens infections in susceptible people.[32] In contrast, Deferiprone is a chelator that has been found to have higher tissue penetration, which allows the medicine to reach more difficult locations and is more effective against infections given its antibacterial properties.[33,34] In this study, patients receiving chelation therapy, particularly combined therapy, had a significantly lower risk of infection (OR 0.18, p<0.001). This may reflect the chelator’s role in reducing iron burden. However, the observed protective effect could also be influenced by better patient follow-up and adherence, as combined therapy often necessitates more frequent monitoring. For instance, regular clinic visits may improve adherence to vaccination schedules and antibiotic prophylaxis, further reducing infection risk.[35] These findings highlight the need for closely monitoring and tailoring treatment techniques in chelation therapy, as each patient's response to treatment may differ; hence, the treatment plan should be tailored to each patient.[36] It also highlights the need for longitudinal studies that can better isolate the effects of chelators from those of follow-up intensity and compliance.
The spleen plays a vital role in immune defense by initiating responses to blood-borne antigens, producing antibodies, and removing antibody-coated pathogens. It contains a diverse population of cells that contribute to both innate and adaptive immunity.[37] Therefore, splenectomy is known to be associated with an increased risk of infections.[22] Our study reinforces this associated risk, demonstrating that individuals who have undergone splenectomy exhibit a higher risk of infection, although this finding did not reach statistical significance. While clear vaccination guidelines exist for splenectomized patients, recommendations for vaccination against encapsulated organisms in non-splenectomized patients with thalassemia are less specific,[35] leading to variation in clinical practice. The lower observed infection rates among vaccinated patients in our study underscore the importance of immunization. Notably, no encapsulated organisms were identified among the detected pathogens.
Recent research has found a substantial correlation between low serum albumin levels and increased infection rates in thalassemia patients. Hypoalbuminemia is linked to the onset and severity of infectious illnesses, and intact innate and adaptive immune responses rely on albumin.[25] Previous research demonstrated that persons with thalassemia have significantly lower albumin levels than those who are healthy.[38,39] This was explained by increased urinary albumin excretion in thalassemia patients, which is caused by chronic anemia/hypoxia with rapid RBC turnover, IOL, and nephrotoxic iron chelators, all of which led to impaired renal tubular reabsorption and increased urine loss.[40] This change has the potential to increase patients' susceptibility to infection, highlighting a need for more study and heightened clinical awareness. The findings suggest that serum albumin may be an essential measure for determining infection risk in this group.
Escherichia coli was the most common bacterium causing bloodstream and urinary tract infections. These findings are consistent with a recent Saudi study that identified Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae as the primary sources of infection in thalassemia patients.[18] In contrast, a study conducted in Taiwan identified Klebsiella pneumoniae as the most common pathogen, with Pseudomonas aeruginosa also playing a substantial role.[20] Interestingly, none of the patients in our study tested positive for additional "iron-loving" infections such as Mucormycosis, Listeria monocytogenes, Yersinia enterocolitica, Aeromonas hydrophila, Cunninghamella bertholletiae, or Vibrio vulnificus.[26] There is no indication in vivo that "iron-loving" pathogen-related infections are more common or severe in thalassemia patients than in the general population.[26]
Patients with positive cultures had a much greater mortality rate than those without (22% vs. 3%). Most of these individuals had serious infections that required an ICU stay. These severe infections, which are frequently aggravated by iron overload, can cause life-threatening diseases and considerably contribute to the mortality rate of thalassemia patients. In line with our findings, a prior study conducted at our institution found that between 12% and 26% of mortality in thalassemia patients was caused by severe consequences from iron overload, including infections.[18,41,42] This emphasizes the essential need for better infection prevention and management measures in thalassemia patients.
In the current investigation, patients with HBH had the highest probability of having a culture-positive result when compared to thalassemia syndromes. This link between HBH in thalassemia patients and infection was previously documented.[43,44] Hemolytic crises are a common clinical characteristic of α-thalassemia and other HBH disorders. It is characterized by an immediate worsening of anemia, which is a risk factor for infection.[43]
Our study was advantageous in that it was representative of a geographical location known to have a high prevalence of thalassemia and included a relatively large number of patients. It provides insight into the epidemiology and risk factors of infection in thalassemia patients in a tertiary center in Saudi Arabia. It emphasizes the importance of close monitoring of iron overload, optimizing chelation therapy to reduce iron burden, and ensuring adherence to recommended vaccination schedules. However, it had drawbacks, notably that it was a retrospective analysis with some missing data and the inability to control for several potential confounding factors, such as the patient's socioeconomic status and the presence of other comorbid conditions. We believe addressing these factors might have led to an even higher estimated infection rate. Furthermore, there are some inherent limits to looking at infections, as some infections are non-culturable, increasing the likelihood of under-detecting infection rates. Larger prospective studies are required to further investigate the findings.
Conclusions
In this retrospective study, we observe a noteworthy prevalence of infections, with a positive culture occurring in approximately 23.5% of patients and a significant association between iron overload and increased infection risk, underscoring its detrimental effect on the immune function in thalassemia patients. Moreover, the study suggests that adherence to chelation therapy (especially combined therapy) may confer a protective effect against infections, highlighting the importance of effective iron management in thalassemia care. However, this deserves further exploration in larger studies. Overall, the study highlights the complex interplay between thalassemia management and infection risk with implications for clinical practice. Continued efforts to optimize chelation therapy, monitor iron overload, and address other specific needs of vulnerable subgroups are essential in improving the health outcomes of thalassemia patients. Further studies should focus on longitudinal studies to further elucidate these relationships.References
- Khan I, Shaikh H. Beta Thalassemia Major (Cooley Anemia).
StatPearls. Treasure Island (FL): StatPearls Publishing Copyright ©
2025, StatPearls Publishing LLC.; 2025.
- Rao E, Kumar
Chandraker S, Misha Singh M, Kumar R. Global distribution of
β-thalassemia mutations: An update. Gene. 2024;896:148022.
https://doi.org/10.1016/j.gene.2023.148022 PMid:38007159
- Mangla
A EM, Agarwal N, et al. . Sickle Cell Anemia. Treasure Island (FL):
StatPearls Publishing; 2023 [Available from: Available from:
https://www.ncbi.nlm.nih.gov/books/NBK482164/]
- Olivieri NF, Pakbaz
Z, Vichinsky E. Hb E/beta-thalassaemia: a common & clinically
diverse disorder. Indian J Med Res. 2011;134(4):522-31.
- Hunnuan
I, Sanpkit K, Lertbannaphong O, Buaboonnam J. Hemoglobin H Disease and
Growth: A Comparative Study of DHbH and NDHbH Patients. Mediterr J
Hematol Infect Dis. 2023;15(1):e2023045.
https://doi.org/10.4084/MJHID.2023.045 PMid:37705526 PMCid:PMC10497309
- Viprakasit V, Ekwattanakit S. Clinical Classification,
Screening and Diagnosis for Thalassemia. Hematol Oncol Clin North Am.
2018;32(2):193-211. https://doi.org/10.1016/j.hoc.2017.11.006
PMid:29458726
- Patterson S, Singleton A, Branscomb J, Nsonwu
V, Spratling R. Transfusion Complications in Thalassemia: Patient
Knowledge and Perspectives. Front Med (Lausanne). 2022;9:772886.
https://doi.org/10.3389/fmed.2022.772886 PMid:35299838 PMCid:PMC8923080
- Lal A, Wong T, Keel S, Pagano M, Chung J, Kamdar A, et al.
The transfusion management of beta thalassemia in the United States.
Transfusion. 2021;61(10):3027-39. https://doi.org/10.1111/trf.16640
PMid:34453453 PMCid:PMC9292563
-
Pinto VM, Forni GL.
Management of Iron Overload in Beta-Thalassemia Patients: Clinical
Practice Update Based on Case Series. Int J Mol Sci. 2020;21(22).
https://doi.org/10.3390/ijms21228771 PMid:33233561 PMCid:PMC7699680
- Cianciulli P. Iron chelation therapy in thalassemia
syndromes. Mediterr J Hematol Infect Dis. 2009;1(1):e2009034.
https://doi.org/10.4084/mjhid.2009.0034
- Atmakusuma TD, Girson R,
Koesnoe S. Correlations between Iron Load and CD4 in Adult
Transfusion-Dependent Beta Thalassemia. Anemia. 2021;2021(1):5549503.
https://doi.org/10.1155/2021/5549503 PMid:34239727 PMCid:PMC8233081
- Vento S, Cainelli F, Cesario F. Infections and
thalassaemia. Lancet Infect Dis. 2006;6(4):226-33.
https://doi.org/10.1016/S1473-3099(06)70437-6 PMid:16554247
- Sharma
A, Easow Mathew M, Puri L. Splenectomy for people with thalassaemia
major or intermedia. Cochrane Database Syst Rev. 2019;9(9):Cd010517.
https://doi.org/10.1002/14651858.CD010517.pub3 PMid:31529486
- Tahir
F, Ahmed J, Malik F. Post-splenectomy Sepsis: A Review of the
Literature. Cureus. 2020;12(2):e6898.
https://doi.org/10.7759/cureus.6898
- Luu S, Spelman D,
Woolley IJ. Post-splenectomy sepsis: preventative strategies,
challenges, and solutions. Infect Drug Resist. 2019;12:2839-51.
https://doi.org/10.2147/IDR.S179902 PMid:31571940 PMCid:PMC6748314
- Alqahtani MM AA, Asirri SA, Alwuthaynani MT, Ishaq YMA,
Hasan ER, Almasri WA, Alkhalil AM, Murdhimah AHA, Albalawai IN.
Prevalence of thalassemia in Saudi Arabia: a systematic review and
meta-analysis. IJMDC. 2024;8(10):2903-12.
https://doi.org/10.24911/IJMDC.51-1729873974
- Connor L, Dean J,
McNett M, Tydings DM, Shrout A, Gorsuch PF, et al. Evidence-based
practice improves patient outcomes and healthcare system return on
investment: Findings from a scoping review. Worldviews Evid Based Nurs.
2023;20(1):6-15. https://doi.org/10.1111/wvn.12621 PMid:36751881
- Alzhrani
FS, Algethmi FA, Makin MA, Barayan NA, Hilal RM, Alnakhli SM.
Prevalence and risk factors of severe bacterial infections in
thalassemia patients. Current Pediatric Research. 2020;24:264-9.
- Ahmed
Kiani R, Anwar M, Waheed U, Asad MJ, Abbasi S, Abbas Zaheer H.
Epidemiology of Transfusion Transmitted Infection among Patients with
β-Thalassaemia Major in Pakistan. J Blood Transfus. 2016;2016:8135649.
https://doi.org/10.1155/2016/8135649 PMid:27559490 PMCid:PMC4983372
- Wang SC, Lin KH, Chern JP, Lu MY, Jou ST, Lin DT, et al.
Severe bacterial infection in transfusion-dependent patients with
thalassemia major. Clin Infect Dis. 2003;37(7):984-8.
https://doi.org/10.1086/378062 PMid:13130412
-
Sakran W,
Levin C, Kenes Y, Colodner R, Koren A. Clinical spectrum of serious
bacterial infections among splenectomized patients with
hemoglobinopathies in Israel: a 37-year follow-up study. Infection.
2012;40(1):35-9. https://doi.org/10.1007/s15010-011-0178-5
PMid:21866338
- Teawtrakul N, Jetsrisuparb A, Sirijerachai C,
Chansung K, Wanitpongpun C. Severe bacterial infections in patients
with non-transfusion-dependent thalassemia: prevalence and clinical
risk factors. Int J Infect Dis. 2015;39:53-6.
https://doi.org/10.1016/j.ijid.2015.09.001 PMid:26358855
- Scott
CR, Holbein BE, Lehmann CD. Iron should be restricted in acute
infection. Front Biosci (Landmark Ed). 2020;25(4):673-82.
https://doi.org/10.2741/4827 PMid:31585910
- Rahav G, Volach
V, Shapiro M, Rund D, Rachmilewitz EA, Goldfarb A. Severe infections in
thalassaemic patients: prevalence and predisposing factors. Br J
Haematol. 2006;133(6):667-74.
https://doi.org/10.1111/j.1365-2141.2006.06082.x PMid:16704445
- Wiedermann
CJ. Hypoalbuminemia as Surrogate and Culprit of Infections. Int J Mol
Sci. 2021;22(9). https://doi.org/10.3390/ijms22094496 PMid:33925831
PMCid:PMC8123513
- Cappellini MD, Cohen A, Eleftheriou A,
Piga A, Porter J, Taher A. Guidelines for the Clinical Management of
Thalassaemia. Nicosia (CY): Thalassaemia International Federation ©
2008 Thalassaemia International Federation.; 2008.
- Cherayil
BJ. Iron and immunity: immunological consequences of iron deficiency
and overload. Arch Immunol Ther Exp (Warsz). 2010;58(6):407-15.
https://doi.org/10.1007/s00005-010-0095-9 PMid:20878249
PMCid:PMC3173740
-
Kao JK, Wang SC, Ho LW, Huang SW, Chang
SH, Yang RC, et al. Chronic Iron Overload Results in Impaired Bacterial
Killing of THP-1 Derived Macrophage through the Inhibition of Lysosomal
Acidification. PLoS One. 2016;11(5):e0156713.
https://doi.org/10.1371/journal.pone.0156713 PMid:27244448
PMCid:PMC4886970
- Kumar A, Ye C, Nkansah A, Decoville T,
Fogo GM, Sajjakulnukit P, et al. Iron regulates the quiescence of naive
CD4 T cells by controlling mitochondria and cellular metabolism.
Proceedings of the National Academy of Sciences.
2024;121(17):e2318420121. https://doi.org/10.1073/pnas.2318420121
PMid:38621136 PMCid:PMC11047099
-
Ru Q, Li Y, Chen L, Wu Y,
Min J, Wang F. Iron homeostasis and ferroptosis in human diseases:
mechanisms and therapeutic prospects. Signal Transduction and Targeted
Therapy. 2024;9(1):271. https://doi.org/10.1038/s41392-024-01969-z
PMid:39396974 PMCid:PMC11486532
- Ba T, Zhao D, Chen Y, Zeng
C, Zhang C, Niu S, et al. L-Citrulline Supplementation Restrains
Ferritinophagy-Mediated Ferroptosis to Alleviate Iron Overload-Induced
Thymus Oxidative Damage and Immune Dysfunction. Nutrients. 2022;14(21).
https://doi.org/10.3390/nu14214549 PMid:36364817 PMCid:PMC9655478
- Neilands
JB. Siderophores: structure and function of microbial iron transport
compounds. J Biol Chem. 1995;270(45):26723-6.
https://doi.org/10.1074/jbc.270.45.26723 PMid:7592901
- Kontoghiorghes
GJ, Efstathiou A, Kleanthous M, Michaelides Y, Kolnagou A. Risk/benefit
assessment, advantages over other drugs and targeting methods in the
use of deferiprone as a pharmaceutical antioxidant in iron loading and
non iron loading conditions. Hemoglobin. 2009;33(5):386-97.
https://doi.org/10.3109/03630260903217141 PMid:19814684
- Thompson
MG, Corey BW, Si Y, Craft DW, Zurawski DV. Antibacterial activities of
iron chelators against common nosocomial pathogens. Antimicrob Agents
Chemother. 2012;56(10):5419-21. https://doi.org/10.1128/AAC.01197-12
PMid:22850524 PMCid:PMC3457357
- Cappellini MD, Farmakis, D.,
Porter, J. Taher, A. et al. Guidelines for the Management of
Transfusion-Dependent Thalassaemia. Thalassaemia International
Federation. 2021;4th edition - Version 2.0.
- Karagün BŞ.
ADVANCES IN THALASSEMIA MANAGEMENT AND CHELATION THERAPY. Hematology,
Transfusion and Cell Therapy. 2024;46:S5-S6.
https://doi.org/10.1016/j.htct.2024.11.093
- Lewis SM,
Williams A, Eisenbarth SC. Structure and function of the immune system
in the spleen. Sci Immunol. 2019;4(33).
https://doi.org/10.1126/sciimmunol.aau6085 PMid:30824527
PMCid:PMC6495537
- Shaalan MG, Hassan MK, Al-Shanoof HJ, Al
Naama LM. Renal Dysfunction in Pediatric Patients in Iraq With
β-Thalassemia Major and Intermedia. Cureus. 2022;14(9):e29183.
https://doi.org/10.7759/cureus.29183
- Mahmoud AA, Elian DM,
Abd El Hady NM, Abdallah HM, Abdelsattar S, Khalil FO, et al.
Assessment of Subclinical Renal Glomerular and Tubular Dysfunction in
Children with Beta Thalassemia Major. Children (Basel). 2021;8(2).
https://doi.org/10.3390/children8020100 PMid:33546213 PMCid:PMC7913373
- Demosthenous C, Vlachaki E, Apostolou C, Eleftheriou P, Kotsiafti A, Vetsiou E, et al. Beta-thalassemia: renal complications and mechanisms: a narrative review. Hematology. 2019;24(1):426-38. https://doi.org/10.1080/16078454.2019.1599096 PMid:30947625
- Zurlo MG,
De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, et
al. Survival and causes of death in thalassaemia major. Lancet.
1989;2(8653):27-30. https://doi.org/10.1016/S0140-6736(89)90264-X
PMid:2567801
- Borgna-Pignatti C, Ventola M, Friedman D, Cohen AR, Origa R, Galanello R, et al. Seasonal variation of pretransfusion hemoglobin levels in patients with thalassemia major. Blood. 2006;107(1):355-7. https://doi.org/10.1182/blood-2005-03-1231 PMid:16179377
- Fucharoen S, Viprakasit V. Hb H disease: clinical course
and disease modifiers. Hematology Am Soc Hematol Educ Program.
2009:26-34. https://doi.org/10.1182/asheducation-2009.1.26
PMid:20008179
- Songdej D FS. INFECTIONS AND HAEMOGLOBIN H DISEASE. Amid A LA, Coates TD, et al., editors., editor. Nicosia (Cyprus): Thalassaemia International Federation; 2023.