Meningeal syndromes are also among the most common reasons for consultation in infectious disease emergency departments in Algeria. Its detection requires emergency hospitalisation for a lumbar puncture and cerebrospinal fluid (CSF) analysis to establish an etiological diagnosis. The positive diagnosis of meningeal syndrome is based on the classic triad of symptoms: headache, vomiting, and neck stiffness. In fact, lumbar puncture remains the gold standard for diagnosing acute meningeal syndrome.[9]
In Algeria today, it is essential to consider the balance between the broad spectrum of Rickettsial symptoms and the limited availability of biological diagnostic tools. Consequently, infectious disease specialists often rely primarily on clinical diagnosis, which creates a significant challenge and directly impacts the therapeutic approach. In light of these considerations, our study aims to highlight the correlation between clinical suspicion of rickettsioses and its detection in patients exhibiting confirmed meningeal syndrome, particularly given the overlapping clinical signs, such as fever and headaches.
Material & Methods
Study design. To provide evidence, we investigated the relationship between clinical suspicion of Rickettsioses and its confirmation in patients with meningeal syndrome; we considered it wise to focus our study at the National Reference Centre for Infectious Diseases in Algeria, named ELHADI FLICI Hospital, Nicolle-Laveran department, Algiers, which admits patients from all the Algerian departments. Our study was conducted between July and September 2019, which coincides with the summer season, when vector-borne and tropical diseases are most prevalent. In total, 55 patients from various districts of Algeria were admitted to our hospital and included in the study.Inclusion criteria. To select the patients most suitable for our study, we included only those diagnosed with a Viral Meningeal Syndrome, confirmed clinically with physical meningeal syndrome: Stiff neck and signs of Kernig “Knees extension” and Brudzinski “full flexion of the hips”, and supported by positive findings from a Cerebrospinal Fluid (CSF) analysis: Moderate increase of the White Blood Cell count (50-1000 cells/µL), predominantly lymphocytes, and normal protein or slightly elevated (50-200 mg/dL), this step was realised at the central laboratory of ELHADI FLICI Hospital-Algiers in order to confirm the meningeal syndrome. These patients also needed to exhibit at least three of the following clinical signs indicative of Rickettsial infections: sudden onset, high fever, severe headache, arthralgia, myalgia, tick bites, characteristic rash (maculopapular or purpuric), Black Spot, and a general feeling of illness. A total of 55 patients were enrolled in the study: 14 adult males (25.45%), 22 adult females (40.00%), and 19 children (under 15 years old, 34.54%: 11 males and 9 females); the mean age was 24.03 (2 to 50 years old). In parallel, we completed a questionnaire for each patient to gather additional information, such as epidemiological data, housing area (rural or urban), contact with ticks or animals, profession, and any history of tick bites.
Sample’s collection. A total of 55 whole blood and 55 sera samples belonging to positive Meningeal Syndrome patients were collected aseptically in suitable EDTA and dry tubes, respectively. The samples were conserved at -20°c to be handled at the URMITE (Emerging Tropical Infectious Diseases Research Unit at the Institut Hospitalo-Universitaire (IHU) Marseille) for IFA serology and qpcr for the rickettsial spp.
Serological assays. Serologic tests were performed using an indirect immunofluorescent antibody (IFA) assay, which is the reference method for the serodiagnosis of Rickettsioses. At the serological service of the IHU Marseille, we test a panel of Rickettsial antigens, such as R. conorii, R.felis, and R. typhi. Immunoglobulin G (IgG) antibody titers of 1:128, seroconversion in paired serum specimens, with/not IgM antibody titers of 1:32 against any species, were considered evidence of recent Rickettsia infection.[2]
DNA extraction and real-time PCR. A total of 200 μL of DNA was extracted from whole blood tubes using the QIAamp Tissue Kit by QIAGEN-BioRobot EZ1, according to the manufacturer's instructions (Qiagen, Hilden, Germany). Extracted DNA was stored at -20°C under sterile conditions until it was used in PCR assays. Extracted DNA was used in qPCR amplifications of the gltA gene of Rickettsia species.[9] Positive samples are further amplified by PCR targeting the outer membrane protein (ompB)[10] and citrate synthase (gltA) genes.[11] The final qPCR reaction mixture consisted of 5 μL DNA with 15 μL of mix from the Roche PCR Kit (Roche Diagnostics, Meylan, France). The PCR cycling parameters for the qPCR were 5 min at 95°C followed by 39 cycles, each consisting of 5 sec of denaturation at 95°C and 30 sec of annealing at 60°C.
Results
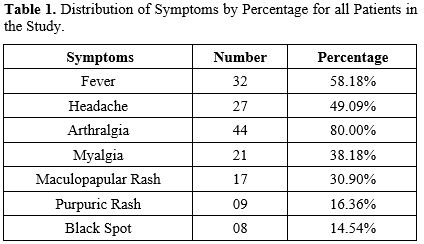
IFA Serology. A total of 55 sera were collected from 55 patients.Table 1 summarises the distribution of signs and symptoms among all patients included in the study.
The results shown in Table 1 lead us to point out that Fever and Arthritis were the most common symptoms for all the patients included in the study (58.18% and 80.00%, respectively). However, only 14.54% of the population study have Black Spots, and 16.36% have a purpuric rash. A maculopapular rash is the most prominent cutaneous symptom of Rickettsial disease; however, we found it in only 30.90% of patients. This variation in symptoms from one patient to another makes a definitive diagnosis challenging.
The screening for all the sera for the antigens against R. conorii, R. felis and R. typhi came back positive for only 07 sera (07/55, 12.72%).
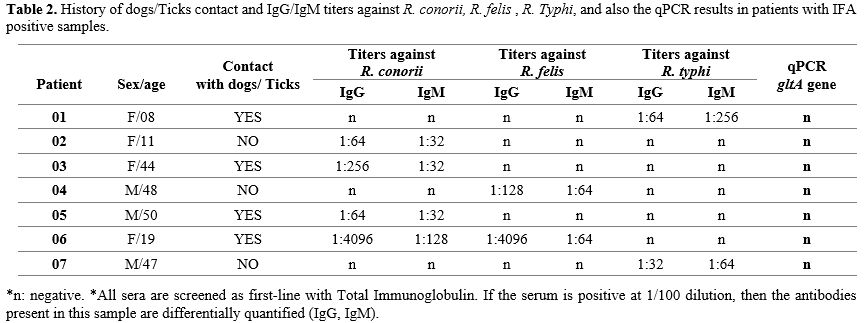
Table 2 reports the IgG and IgM titers against R. conorii, R. felis, and R. typhi, as well as the qPCR results and other signs belonging to positive IFA.
 |
|
Using IFA against Rickettsia spp antigens, we obtained only 07 positive samples from 55 ones (12.72%). Among these positive samples, we note that 04 of them are Female, and the 03 left are male. However, the Dog/Tick contact did not reflect the real infectious status because even with no Dog/Tick contact, we found positive rickettsial titers. Otherwise, according to these IFA results, we diagnosed 04 cases of Spotted Mediterranean Fever (MSF) caused by R. conorii, 02 cases of Murine Typhus caused by R. typhi, and only one (01) case of Flea-borne Spotted Fever caused by R. felis. In parallel, the IFA of patient number 06 came back positive for two (02) Rickettsioses, R. conorii and R. felis. These cross-reactive antibodies have been confirmed by the Western-Blot test, which makes the result more sensitive in support of R. conorii positivity.
The four cases of MSF and one case of Flea-borne Spotted Fever belong to the SFG of Rickettsial diseases; however, the two cases of Murine Typhus belong to the TG of Rickettsioses.
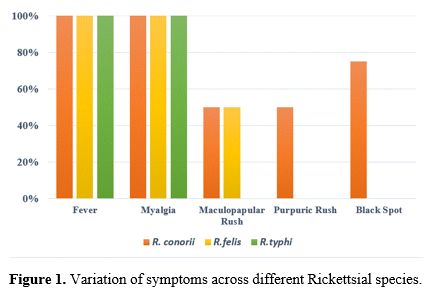
Regarding the positive results obtained and the variation in symptoms among these patients, as shown in Figure 1.
In this analysis, we examine the results based on symptoms and the bacterial species involved. Fever and myalgia are consistently observed in all positive patients, regardless of the bacterial species, indicating that these are common symptoms of Rickettsial infections. Additionally, a maculopapular rash is present in half of the patients with both species of R. conorii and R. felis but is absent in those infected with R. Typhi. Nevertheless, purpuric rash is observed in 50% of cases with R. conorii but is missing in patients with other strains of R. conorii and R. Typhi. The feature black spot is found exclusively in cases of R. conorii. By recapping, all Rickettsia-positive patients exhibit fever and myalgia, while skin lesions are observed only in patients positive for R. conorii and R. felis. However, the Tache noire sign is present solely in patients positive for R. conorii.
This distribution of symptoms suggests that while fever and myalgia are general indicators of Rickettsial infections, the presence of specific symptoms such as maculopapular rash, purpuric rash, and tache noire might help in differentiating between various Rickettsial species and strains. This information is essential for accurate diagnosis and appropriate treatment.
Detection of Rickettsia spp by quantitative qPCR. qPCR was used to detect Rickettsia spp in whole blood by employing Rickettsia-specific primers and a probe designed to amplify the gltA gene. All the whole blood qPCR came back negative for the Rickettsial gene gltA, even in patients whose sera tested positive for any Rickettsial species using IFA. These results could be explained by the samples not coinciding with the Rickettsiae bacteraemia phase.
On the other hand, the Complete Blood Count (CBC) of all the positive rickettsial cases was represented by hyperleukocytosis. Also, for all of them, no thrombocytopenia was observed, and C-reactive protein (CRP) was normal. Concerning the Spinal Tap, the Cerebrospinal Fluid aspect was clear in 04 rickettsial cases (01 R.conorii, 02 R.felis and 01 R.typhi). However, 03 R.conorii showed an opalescent Cerebrospinal Fluid aspect. In the cytology test, the mean was 737 elements/mm3 for the rickettsial cases, with 03 lymphocytic cases (02 R.felis and 01 R.typhi) and 04 mixed cellular cases (04 R.conorii). In addition, we had a slight elevation of Cerebrospinal Fluid Proteins and normal glycorrhachia.
Discussion
Aiming to showcase the relationship between clinical suspicion of Rickettsioses in patients with confirmed meningeal syndrome, we focused our study at the National Centre of Infectious Diseases EL-HADI FLICI Hospital in Algiers. During the study period, from July to October 2019, high temperatures were recorded in Algiers (till 41.5 °C); knowing that Algeria is a warm country, vector-borne infectious diseases are widespread in the summer season. It is well known that the distribution of vectors and associated pathogen transmission rates can be affected by changes in ambient temperature and climate.[1] Therefore, other species of ticks are getting into the Rickettsial cycle. Thus, the infected blood of these vectors potentially increases the risks of human infection.Mariusz Piotrowski and colleagues (2020) reported that the discovery of new rickettsiae has altered our understanding of the clinical features and epidemiology of these bacteria. In addition to the classic symptomatic triad of fever, rash, and headache, it has been demonstrated that each rickettsiosis possesses distinct characteristics, including severity and inoculation rate.[12]
Moreover, the meningeal syndrome is a set of symptoms associated with the pathological irritation of the meningeal envelopes of the central nervous system; this disease was confirmed clinically and biologically by all patients included in our study. Furthermore, a panel of clinical symptoms in favour of Rickettsial infections was required for the inclusion criteria, such as fever, severe headache, a characteristic rash (maculopapular, purpuric), and a general feeling of illness (malaise). A total of 55 sera belonging to 55 patients were collected aseptically and tested by IFA Rickettsial serology of Rickettsia spp. Rickettsial IFA serology came back positive for four 07 sera from the 55 tested (12.72%). The positivity of Rickettsial IFA varies from patient to patient, with precisely 04 cases of Spotted Mediterranean Fever (MSF), 02 cases of Murine Typhus, and only one (01) case of the Flea-borne Spotted Fever. The positive patient’s sera for Rickettsioses and the confirmed meningeal syndromes in all these cases, on the one hand, and the various clinical manifestations of Rickettsial and meningeal symptoms among these patients, on the other hand, lead us to suggest the competitive physio-pathogenicity expression between the two pathogens. All positive cases exhibit symptoms of fever or more clinical findings. The most common finding was the presence of Maculopapular Rash and Myalgia. This different expression of clinical symptoms for both pathogens (Meningeal and Rickettsial) depends on the severity of the disease and may range from mild to more severe. It is noteworthy to assess these results concerning the co-infections between the Meningeal pathogen and the Rickettsia spp. Moreover, the absence of some key clinical features (for MSF diagnosis) in our patients confirms the clinical polymorphism. It makes the diagnosis difficult, which is supposed to be clinical, leaving the morbidity underestimated in endemic areas. In other words, negative inflammatory assessment is a characteristic of intracellular bacteria. Confirmation is useful for seroprevalence studies but remains late, giving a retrospective diagnosis of interest for knowledge of the clinical description of rickettsioses in general (particularly R. felis) and without therapeutic support. This late diagnosis, which results in late treatment or even no treatment at all, is considered to be a factor with a poor prognosis.
Regarding the spinal tap, the cerebrospinal fluid appeared opalescent in the three patients who tested positive for R. conorii. Additionally, the cytology test revealed a mixed cellular profile in these three patients. Based on these findings, the opalescent appearance and the mixed cell profile may indicate the presence of a co-infection (in this case, with R. conorii) in patients presenting with meningeal syndrome.
In 2019, a study was conducted in South Africa to assess the laboratory diagnostic capacity for Spotted Fever Rickettsiosis. The study reported that there was no standardised testing platform or interpretation for serological tests across the various laboratories involved. Additionally, PCR tests were less frequently available. This lack of standardisation and availability poses a significant challenge for confirming diagnoses and managing cases, particularly given the multitude of potential differential diagnoses.[12]
In addition, further studies also took into account the Rickettsial disease. In Western Algeria, Oran, the first cases of MSF were clinically diagnosed for the first time in 1993, and since that time, the number of cases has steadily increased to reach 134 in 2004. Mouffok et al. in 2006, conducted a study in Western Algeria in order to present the descriptive clinic and epidemiology to identify more severe forms, the presence of multiple eschars, and different rickettsial strains caused the disease, where they found 63 positive IFA Rickettsial serology from out 104 sera, and they also reported that all the patients included in the study had fever, and 44% of them presented underlying diseases, and 72% of the population study have a Maculopapular rash.[8] All the same, Mouffok et al., in 2009, reported 24 positive IFA serology for R. conorii (24/36), belonging to Algerian children’s patients. These results are in concordance with ours in terms of the clinical expression of Rickettsial disease and especially the presence of an underlying infection.[13] In addition, Mouffok et al., in 2011, evaluated the advantage of skin swab samples (Black Spot) for the diagnosis of Rickettsial diseases in Algeria by a molecular detection (qPCR) of the RC0338 gene which is specific to Rickettsia spp; they obtained 63.4% of positive samples, these results confirmed that the efficiency of the skin swab sampling to diagnose the Rickettsioses, and also confirmed the presence of the Rickettsial pathogen in Algeria.[14] Another study was conducted by Mokrani et al in 2012, aiming to have a prevalence of Rickettsioses in Febrile Exanthemas in Eastern Algeria, where they calculated 12.96% of positive IFA serology confirmed by Western Blot within 5 cases of R. conorii, 2 cases of R. felis and 4 cases of R. typhi, the left 03 cases belonged to other Rickettsia species (R. aeschlimannii).[6] In a common epidemiological study of Rickettsia felis and Malaria in Africa, Mediannikov et al. in Africa (2013) worked for a cohort of African patients: 266 patients from Western Algeria, 183 patients from Tunisia and 48 patients from Morocco, and also 400 patients from Marseille as control patients. They reported 02 positive blood qPCR for R. conorii and 01 positive blood qPCR for R. conorii in Algerian and Moroccan patients, respectively. However, even Tunisia and Marseille have no positive case of R. conorii. These results confirm the linear relationship between Rickettsioses and other underlying infections (Malaria in this case), as we reported in our study between Rickettsioses and Meningeal Syndrome.
Nevertheless,[2] Znazen et al., in 2006, in Tunisia, reported 86 positive IFA serology for R. felis confirmed by Western Blot from out 638 patients' sera tested.[15] In addition, in Greece, Chochlakis et al., in 2017, conducted a study aimed at improving testing of tick-borne diseases; they got 28 positive IFA for R. typhi and eight positive IFA for R. felis. Rickettsial diseases remain a born-vector disease, mainly transmitted by arthropods, such as ticks, fleas, body lice and mites.[1]
Rickettsia spp are widely distributed across the Mediterranean region, a geographic area characterised by diverse ecological landscapes that facilitate the survival and dissemination of these obligate intracellular bacteria. The seroprevalence of Rickettsia spp. in Mediterranean countries varies widely, ranging from approximately 8% to 30%.[16] In Greece, serological data indicate rates between 8% and 20%, and this is especially true in rural communities and among individuals with suspected rickettsial illnesses.[17] Moreover, in Turkey, studies have reported seroprevalence rates around 15-25%, particularly among livestock handlers and rural residents, which highlights occupational exposure risks.[18] Similarly, in southern Italy and Spain, human seroprevalence can range from about 10% to 30%, with higher rates observed in populations with greater exposure to tick-infested environments or rural settings.[19] Furthermore, in Morocco, the available data suggest seroprevalence rates around 5-10% among rural populations, reflecting a lower yet still notable level of exposure.[16]
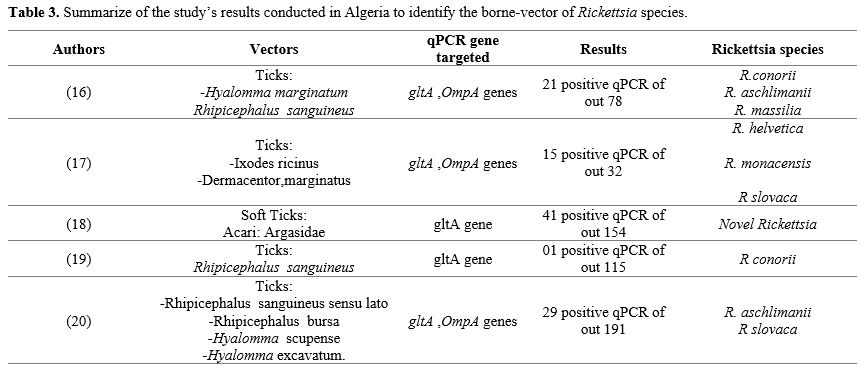
Several studies have been conducted in Algeria to determine the various vectors and reservoirs of the Rickettsioses disease. Table 3 summarises the results of these studies to identify the borne vector of Rickettsia species in Algeria.
 |
|
The previous description of Table 3 suggests the circulation of many Rickettsia spp in Algeria, transmitted mainly by ticks, which remain very important vectors of Rickettsial diseases, especially if they are transported by animal species where humans live.
Globalisation and climate change are factors that can contribute to the increased transmission of rickettsioses by ticks. Rising temperatures can enhance tick aggressiveness and their propensity to bite humans, while on the other hand, they can lead to infections occurring at atypical times of the year. Furthermore, the growing trend of travelling to increasingly exotic destinations raises the likelihood of exposure to ticks that transmit diseases, including rickettsioses.[25]
Conclusions
Based on serological findings and the reported clinical and epidemiological antecedents, there is clear evidence of the existence of human Rickettsial diseases in Algiers, especially with underlying infection. As we explained previously, both rickettsial diseases and meningeal syndromes could simultaneously infect humans, and the clinical expression reflects the severity of the pathogens in question. These findings would provide more attention for the infectious disease specialists in front of a confirmed Meningeal Syndrome, which may have a clinical similarity with the Rickettsial diseases, especially in countries where the hematophagous arthropod-borne zoonosis is highest. Moreover, Polymorphic diseases should, therefore, be suspected even in the absence of a rash.Acknowledgements
The authors thank all the Staff of the IHU-Marseille, France, and all the medical staff of the Algerian National Centre of Infectious Diseases, EL-HADI FLICI Hospital, Nicolle-Lavran department.Ethics approval
The medical committee has approved the collection of samples from the patients included in this study. In addition, all patients provided consent to be included in the study, allowing us to collect interview information and blood samples. Clinical data were obtained through a standardised questionnaire, which included clinical information, contact with animals, and health history.Author contributions
GH conceptually designed the strategy for this study, GH and AN collected samples, GH analysed the samples in the laboratory, IT and NI advised on statistical analyses, GH, AN, and TS drafted the manuscript, and BI and NB analysed the data and corrected the manuscript.References
- Chochlakis, D.; Germanakis, A.; Chaliotis, G.;
Kapetanaki, S.; Kalogeraki, L.; Gkika, E.; Partalis, N.; Polymili, G.;
Tselentis, Y.; Psaroulaki, A. Potential Exposure of Humans to
Rickettsia Felis in Greece. Acta Tropica 2018, 178 (May 2017), 40-45. https://doi.org/10.1016/j.actatropica.2017.10.020 PMid:29079185
- Mediannikov,
O.; Socolovschi, C.; Edouard, S.; Fenollar, F.; Mouffok, N.; Bassene,
H.; Diatta, G.; Tall, A.; Niangaly, H.; Doumbo, O.; Lekana-Douki, J.
B.; Znazen, A.; Sarih, M.; Ratmanov, P.; Richet, H.; Ndiath, M. O.;
Sokhna, C.; Parola, P.; Raoult, D. Common Epidemiology of Rickettsia
Felis Infection and Malaria, Africa. Emerging Infectious Diseases 2013,
19 (11), 1775-1783. https://doi.org/10.3201/eid1911.130361 PMid:24188709 PMCid:PMC3837673
- Walker,
D. H.; Ismail, N. Emerging and Re-Emerging Rickettsioses: Endothelial
Cell Infection and Early Disease Events. Nature Reviews Microbiology
2008, 6 (5), 375-386. https://doi.org/10.1038/nrmicro1866 PMid:18414502
- Kantsø,
B.; Svendsen, C. B.; Jørgensen, C. S.; Krogfelt, K. A. Evaluation of
Serological Tests for the Diagnosis of Rickettsiosis in Denmark.
Journal of Microbiological Methods 2009, 76 (3), 285-288. https://doi.org/10.1016/j.mimet.2008.12.012 PMid:19162092
- La
Scola, B.; Raoult, D. Laboratory Diagnosis of Rickettsioses: Current
Approaches to Diagnosis of Old and New Rickettsial Diseases. Journal of
Clinical Microbiology 1997, 35 (11), 2715-2727. https://doi.org/10.1128/jcm.35.11.2715-2727.1997 PMid:9350721 PMCid:PMC230049
- Mokrani,
K.; Tebbal, S.; Raoult, D.; Fournier, P. E. Human Rickettsioses in the
Batna Area, Eastern Algeria. Ticks and Tick-borne Diseases 2012, 3
(5-6), 364-366. https://doi.org/10.1016/j.ttbdis.2012.10.017 PMid:23182273
- Prata,
J. A. C.; de Souza, C. E.; Angerami, R. N.; Barbosa, T. M. C. D. M.;
dos Santos, F. C. P.; Colombo, S.; del Guercio, V. M. F.; Donalísio, M.
R. Antibodies for Rickettsia Spp. In Patients with Negative Serology
for Dengue Virus, Leptospirosis, and Meningococcal Disease in
Municipalities of São Paulo State, Brazil. Revista da Sociedade
Brasileira de Medicina Tropical 2016, 49 (5), 567-571. https://doi.org/10.1590/0037-8682-0023-2016 PMid:27812650
- Mouffok,
N.; Benabdellah, A.; Richet, H.; Rolain, J. M.; Razik, F.; Belamadani,
D.; Abidi, S.; Bellal, R.; Gouriet, F.; Midoun, N.; Brouqui, P.;
Raoult, D. Reemergence of Rickettsiosis in Oran, Algeria. Annals of the
New York Academy of Sciences 2006, https://doi.org/10.1196/annals.1374.033 PMid:17114705
- Stenos
J, Graves SR, U. N. A Highly Sensitive and Specific Real-Time PCR Assay
for the Detection of Spotted Fever and Typhus Group Rickettsiae. Am J
Trop Med Hyg 2005, 73 (6), 1083-1085. https://doi.org/10.4269/ajtmh.2005.73.1083 PMid:16354816
- Roux,
V.; Raoult, D. Phylogenetic Analysis of Members of the Genus Rickettsia
Using the Gene Encoding the Outer-Membrane Protein ROmpB (OmpB).
International Journal of Systematic and Evolutionary Microbiology 2000,
50 (4), 1449-1455. https://doi.org/10.1099/00207713-50-4-1449 PMid:10939649
- Roux,
V.; Rydkina, E.; Eremeeva, M.; Raoult, D. Citrate Synthase Gene
Comparison, a New Tool for Phylogenetic Analysis, and Its Application
for the Rickettsiae. International Journal of Systematic Bacteriology
1997, 47 (2), 252-261. https://doi.org/10.1099/00207713-47-2-252 PMid:9103608
- Trataris-Rebisz,
A. N.; Rossouw, J.; Markotter, W.; Frean, J. A.; Weyer, J. Spotted
Fever Rickettsiosis in South Africa: Evaluation of Laboratory
Diagnostic Capacity and Inter-Laboratory Comparison of Serological
Testing. South African Medical Journal 2019, 109 (4), 223-226. https://doi.org/10.7196/SAMJ.2019.v109i4.13788 PMid:31084685
- Mouffok,
N.; Parola, P.; Abdennour, D.; Aouati, A.; Razik, F.; Benabdellah, A.;
Raoult, D. Mediterranean Spotted Fever in Algerian Children. Clinical
Microbiology and Infection 2009, 15 (SUPPL. 2), 290-291. https://doi.org/10.1111/j.1469-0691.2008.02241.x PMid:19438656
- Mouffok,
N.; Socolovschi, C.; Benabdellah, A.; Renvoisé, A.; Parola, P.; Raoult,
and D. Diagnosis of Rickettsioses from Eschar Swab Samples, Algeria.
Emerging Infectious Diseases 2013, 13 (11), 1775-1783.
- Znazen,
A.; Rolain, J. M.; Hammami, N.; Hammami, A.; Jemaa, M. Ben; Raoult, D.
Rickettsia Felis Infection, Tunisia. Emerging Infectious Diseases 2006,
12 (1), 138-140. https://doi.org/10.3201/eid1201.050876 PMid:16494731 PMCid:PMC3291393
- Meskini,
M.; Beati, L.; Benslimane, A.; Raoult, D. Seroepidemiology of
Rickettsial Infections in Morocco. European Journal of Epidemiology
1995, 11 (6), 655-660. https://doi.org/10.1007/BF01720299 PMid:8861849
- Dougas,
G.; Tsakris, A.; Billinis, C.; Beleri, S.; Patsoula, E.;
Papaparaskevas, J. Molecular Detection of Rickettsia Felis in Common
Fleas in Greece and Comparative Evaluation of Genotypic Methods.
Journal of Microbiological Methods 2021, 180 (September 2020), 106104. https://doi.org/10.1016/j.mimet.2020.106104 PMid:33217484
- Demir,
S.; Erkunt Alak, S.; Köseoğlu, A. E.; Ün, C.; Nalçacı, M.; Can, H.
Molecular Investigation of Rickettsia Spp. and Francisella Tularensis
in Ticks from Three Provinces of Turkey. Experimental and Applied
Acarology 2020, 81 (2), 239-253. https://doi.org/10.1007/s10493-020-00498-y PMid:32394036
- Guccione,
C.; Colomba, C.; Tolomeo, M.; Trizzino, M.; Iaria, C.; Cascio, A.
Rickettsiales in Italy. Pathogens 2021, 10 (2), 1-27. https://doi.org/10.3390/pathogens10020181 PMid:33567793 PMCid:PMC7915787
- Bitam,
I.; Parola, P.; Matsumoto, K.; Rolain, J. M.; Baziz, B.; Boubidi, S.
C.; Harrat, Z.; Belkaid, M.; Raoult, D. First Molecular Detection of R.
Conorii, R. Aeschlimannii, and R. Massiliae in Ticks from Algeria.
Annals of the New York Academy of Sciences 2006, 1078 (33), 368-372. https://doi.org/10.1196/annals.1374.073 PMid:17114743
- Kernif,
T.; Messaoudene, D.; Ouahioune, S.; Parola, P.; Raoult, D.; Bitam, I.
Spotted Fever Group Rickettsiae Identified in Dermacentor Marginatus
and Ixodes Ricinus Ticks in Algeria. Ticks and Tick-borne Diseases
2012, 3 (5-6), 380-381. https://doi.org/10.1016/j.ttbdis.2012.10.012 PMid:23168054
- Lafri,
I.; Leulmi, H.; Baziz-Neffah, F.; Lalout, R.; Mohamed, C.; Mohamed, K.;
Parola, P.; Bitam, I. Detection of a Novel Rickettsia Sp. in Soft Ticks
(Acari: Argasidae) in Algeria. Microbes and Infection 2015, 17 (11-12),
859-861. https://doi.org/10.1016/j.micinf.2015.09.010 PMid:26408401
- Bessas,
A.; Leulmi, H.; Bitam, I.; Zaidi, S.; Ait-Oudhia, K.; Raoult, D.;
Parola, P. Molecular Evidence of Vector-Borne Pathogens in Dogs and
Cats and Their Ectoparasites in Algiers, Algeria. Comparative
Immunology, Microbiology and Infectious Diseases 2016, 45, 23-28. https://doi.org/10.1016/j.cimid.2016.01.002 PMid:27012917
- Leulmi,
H.; Aouadi, A.; Bitam, I.; Bessas, A.; Benakhla, A.; Raoult, D.;
Parola, P. Detection of Bartonella Tamiae, Coxiella Burnetii and
Rickettsiae in Arthropods and Tissues from Wild and Domestic Animals in
Northeastern Algeria. Parasites and Vectors 2016, 9 (1), 1-8. https://doi.org/10.1186/s13071-016-1316-9 PMid:26791781 PMCid:PMC4721140
- Piotrowski, M.; Rymaszewska, A. Expansion of Tick-Borne Rickettsioses in the World. Microorganisms 2020, 8 (12), 1-28. https://doi.org/10.3390/microorganisms8121906 PMid:33266186 PMCid:PMC7760173