 |
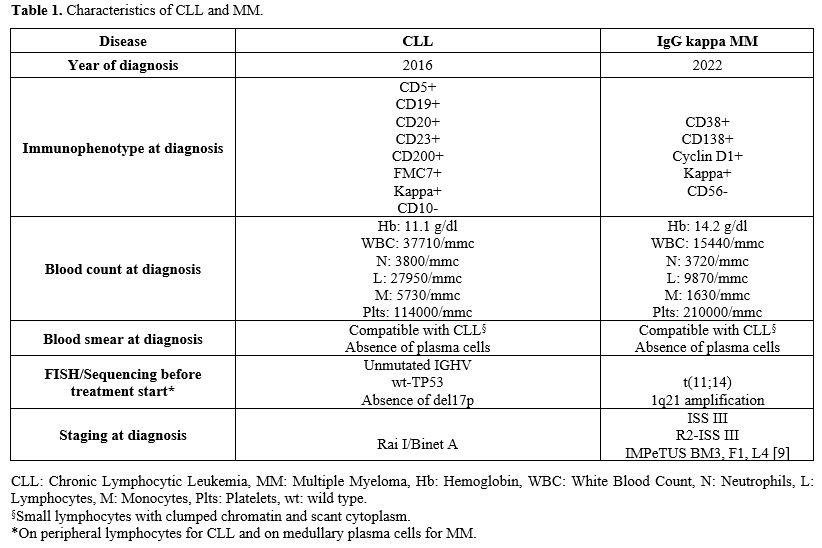
Table 1. Characteristics of CLL and MM. |
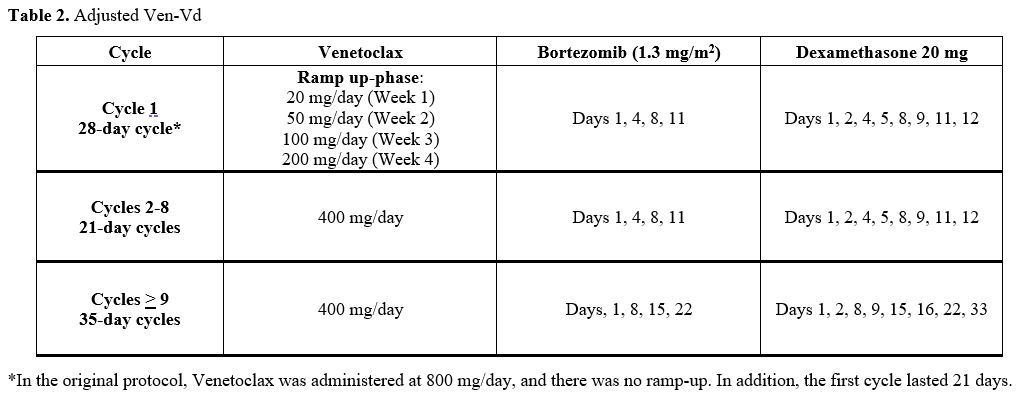 |
Table 2. Adjusted Ven-Vd. |
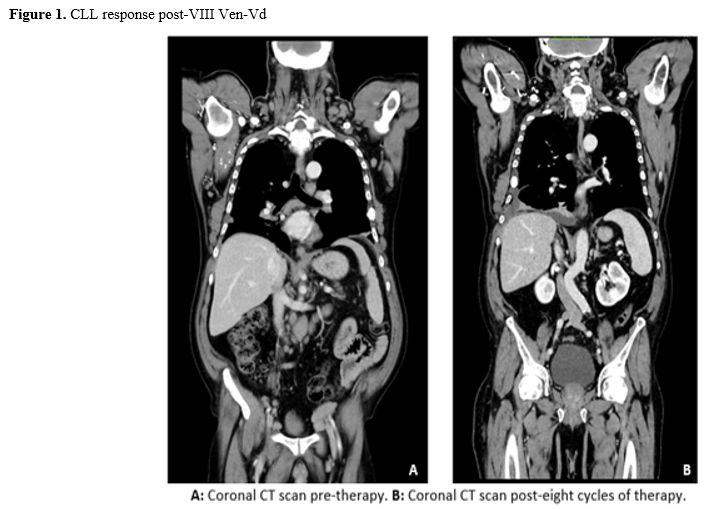 |
Figure 1.CLL response post-VIII Ven-Vd. |
Synchronous and sequential diagnosis of MM and CLL/SLL is a rare event, with a few cases reported in the literature. Multiple studies were conducted in an attempt to prove a clonal relationship between the two diseases. However, different reports suggest that there may not be any clonal relationship between their cells of origin.[2-6] Nevertheless, we can hypothesize that some genes may have pleiotropic effects, and certain biological pathways may affect their mutual development due to the enrichment of B cell regulatory elements.[7-8]
The largest experience in the management of concomitant MM and CLL is reported by Ailawadhi S. et al.[8] From their experience, out of 10735 patients diagnosed with MM between 2000 and 2015, 28 (0.26%) also developed CLL: 15 before the diagnosis of MM, 11 simultaneously, and 2 after. None of them needed specific treatment for CLL, which resulted in responding to anti-myeloma treatment, and their prognosis was not statistically different from patients affected by MM only (58 vs 84 months, p = 0.198). Of note, in their experience, in 14 patients, MM and CLL were restricted to the same light chain. However, they did not attempt to identify a common origin cell or a clonal relationship. No prognostic differences were noted between patients with both diseases, regardless of whether they were restricted to the same light chain or not.
Our experience shows an optimal response for MM and CLL, both with a BELLINI trial-like therapy.[1] The reason for choosing this treatment regimen is the better results observed in relapsed/refractory MM (RRMM) carrying the t(11;14) (PFS 36.8 vs 23.4 months). The Venetoclax maximum dose was reduced (400 mg/day vs 800 mg/day), and a CLL-like ramp-up was performed in our case because of the high risk of hematological and infectious adverse events. In October 2023, we had to withhold Bortezomib for grade 3 peripheral neuropathy, and MM relapsed 14 months later, while CLL response had been retained.
We can conclude that, as already described, MM seems to be the main determinant of survival of this rare subgroup of patients. Ven-Vd could be a promising regimen in this setting. However, more data regarding safety and effectiveness are required.
References
- Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat
R, Gasparetto C, Hungria V, Salwender H, Suzuki K, Kim I, Punnoose EA,
Hong WJ, Freise KJ, Yang X, Sood A, Jalaluddin M, Ross JA, Ward JE,
Maciag PC, Moreau P. Venetoclax or placebo in combination with
Bortezomib and dexamethasone in patients with relapsed or refractory
multiple myeloma (BELLINI): a randomised, double-blind, multicentre,
phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1630-1642. https://doi.org/10.1016/S1470-2045(20)30525-8 PMid:33129376
- Brouet
JC, Fermand JP, Laurent G, Grange MJ, Chevalier A, Jacquillat C,
Seligmann M. The association of chronic lymphocytic leukemia and
multiple myeloma: a study of eleven patients. Br J Haematol. 1985
Jan;59(1):55-66. https://doi.org/10.1111/j.1365-2141.1985.tb02963.x PMid:3882132
- Fermand
JP, James JM, Herait P, Brouet JC. Associated chronic lymphocytic
leukemia and multiple myeloma: origin from a single clone. Blood. 1985
Aug;66(2):291-3. https://doi.org/10.1182/blood.V66.2.291.291 PMid:2990608
- Kaufmann
H, Ackermann J, Nösslinger T, Krömer E, Zojer N, Schreiber S, Urbauer
E, Heinz R, Ludwig H, Huber H, Drach J. Absence of clonal chromosomal
relationship between concomitant B-CLL and multiple myeloma--a report
on two cases. Ann Hematol. 2001 Aug;80(8):474-8.
- Chang
H, Wechalekar A, Li L, Reece D. Molecular cytogenetic abnormalities in
patients with concurrent chronic lymphocytic leukemia and multiple
myeloma shown by interphase fluorescence in situ hybridization:
evidence of distinct clonal origin. Cancer Genet Cytogenet. 2004 Jan
1;148(1):44-8. https://doi.org/10.1016/S0165-4608(03)00217-6 PMid:14697640
- Jamani
K, Duggan P, Neri P, Bahlis N, Jimenez-Zepeda VH. Co-existent B-cell
and plasma cell neoplasms: a case series providing novel clinical
insight. Leuk Lymphoma. 2016;57(3):557-62. https://doi.org/10.3109/10428194.2015.1061189 PMid:26065437
- Law
PJ, Sud A, Mitchell JS, Henrion M, Orlando G, Lenive O, Broderick P,
Speedy HE, Johnson DC, Kaiser M, Weinhold N, Cooke R, Sunter NJ,
Jackson GH, Summerfield G, Harris RJ, Pettitt AR, Allsup DJ, Carmichael
J, Bailey JR, Pratt G, Rahman T, Pepper C, Fegan C, von Strandmann EP,
Engert A, Försti A, Chen B, Filho MI, Thomsen H, Hoffmann P, Noethen
MM, Eisele L, Jöckel KH, Allan JM, Swerdlow AJ, Goldschmidt H, Catovsky
D, Morgan GJ, Hemminki K, Houlston RS. Genome-wide association analysis
of chronic lymphocytic leukaemia, Hodgkin lymphoma and multiple myeloma
identifies pleiotropic risk loci. Sci Rep. 2017 Jan 23;7:41071. https://doi.org/10.1038/srep41071 PMid:28112199 PMCid:PMC5253627
- Ailawadhi
S, Dholaria BR, Khurana S, Sher T, Alegria V, Paulus A, Ailawadhi M,
Mehta A, Chanan-Khan A, Roy V. Outcomes of patients with simultaneous
diagnosis of chronic lymphocytic leukaemia/small lymphocytic lymphoma
and multiple myeloma. Br J Haematol. 2019 Apr;185(2):347-350. https://doi.org/10.1111/bjh.15458 PMid:29978498
- Nanni C. PET-FDG: Impetus. Cancers. 2020; 12(4):1030. https://doi.org/10.3390/cancers12041030 PMid:32331374 PMCid:PMC7226158