Blood culture remains the gold standard for diagnosing NS. However, it presents challenges such as prolonged cycles for result reports and a high false negative rate, which can no longer meet clinical needs.[6] With the advancement of detection technology in recent decades, great concern has been attached to the screening of serological markers as rapid diagnostic tools for clarifying NS pathogens. Although recent studies have also explored the potential of other biomarkers, such as carboxyhemoglobin levels, to predict and manage neonatal sepsis,[7] C-reactive protein (CRP) and procalcitonin (PCT) are still two common auxiliary diagnostic markers for NS, both of which can facilitate the differential diagnosis of bacterial and viral infections.[8] However, their roles in distinguishing between Gram-positive and Gram-negative bacterial infections are still unclear.[9]
Accordingly, the present study retrospectively analyzed the clinical data of newborns with NS who were admitted to our hospital due to Gram-positive and Gram-negative bacterial infections. It aims to investigate the differences in clinical characteristics between Gram-positive and Gram-negative NS and evaluate the significance of CRP, PCT, and PLT in peripheral blood in the differential diagnosis of Gram-positive and Gram-negative bacterial infections.
Materials and Methods
Subjects of study. The study examined 91 newborns who were hospitalized in the Department of Neonatology, Bengbu First People’s Hospital (Bengbu Critical Newborn Treatment Center in Anhui Province) between March 2019 and March 2024, all of whom were diagnosed with bacterial NS via blood culture. According to the Gram staining results, the enrolled newborns with NS were divided into the Gram-negative bacteria group (n=31) and the Gram-positive bacteria group (n=60). Meanwhile, 60 pediatric patients without NS who were hospitalized during the same period were selected as the control group, including those with neonatal hyperbilirubinemia and transient hyperpnea. This study was approved by our hospital's Ethics Committee.Inclusion criteria for 91 NS participants included: (1) newborns born within 28 days after birth; (2) newborns who met the diagnostic criteria for NS in the "Protocol for Diagnosis and Treatment of Neonatal Septicemia", which involved prompt clinical assessment of potential symptoms, immediate blood cultures, empirical broad-spectrum antibiotic therapy based on likely pathogens (usually ampicillin and gentamicin);[10] (3) newborns with a single strain in blood culture; and (4) newborns with complete clinical data. Exclusion criteria included: (1) newborns with negative blood culture; (2) newborns with super-infections or multi-infections; (3) newborns who had used antibiotics before hospitalization; and (4) newborns with severe congenital malformations.
The inclusion criteria of the control group included (1) newborns born within 28 days from birth, (2) newborns with complete clinical data, and (3) negative blood cultures. Exclusion criteria included: (1) newborns with severe congenital malformations; (2) newborns with chromosomal abnormalities; (3) newborns with multi-organ failure and malignant tumors caused by non-infectious diseases; (4) perinatal hypoxia and asphyxia.
Sample size calculation. The sample size for this study was determined based on the primary objective of comparing the diagnostic efficacy of CRP, PCT, and PLT in differentiating between Gram-positive and Gram-negative neonatal sepsis. We assumed a medium effect size (Cohen's d = 0.5) and an alpha level of 0.05 for the differences in CRP, PCT, and PLT levels between the two groups. This effect size was chosen based on previous studies that have reported similar differences in these biomarkers between Gram-positive and Gram-negative infections.[11] Using G*Power software version 3.1.9.7, a post-hoc power analysis was performed. The analysis indicated that our sample size provided a power of 0.82 to detect significant differences between the groups, and that suggests that the study had an 82% chance of correctly rejecting the null hypothesis when the true effect size was 0.5. While a higher power would be ideal, our sample size was sufficient to detect clinically meaningful differences in the biomarker levels between the two groups, given the observed effect sizes in our study.
General data collection. Comprehensive clinical data were collected for all subjects, including gender, gestational age, birth weight, mode of delivery, the occurrence of premature delivery (gestational age <37 weeks), the incidence of premature rupture of membranes (>18 h before delivery), and incidence of amniotic fluid contamination (including hypertension and diabetes). Amniotic fluid contamination was assessed using the NICHD classification system, which grades turbidity into four categories: I (clear), II (moderately stained), III (heavily stained but with visibility of 1 cm), and IV (opaque and non-visual).[12]
Specimen collection and testing,
Blood culture. All pediatric patients were subjected to blood sampling (1-2 mL, venous blood) immediately after admission. Samples were inoculated into BD blood culture flasks and cultured using the BD BACTECTMFX fully automatic blood culture system. Positive specimens were subjected to Gram staining for bacterial strain identification.
Laboratory testing. Venous blood samples were collected within the first 6 hours of admission for the measurement of white blood cell (WBC), neutrophil (NEU), and platelet (PLT) counts using an XE-2100 automatic blood cell analyzer (Sysmex, Japan). CRP levels were determined by rate nephelometry using an AU5800 fully automatic biochemical analyzer (Beckman Coulter, USA), and PCT levels were measured by electrochemiluminescence using a Cobase601 fully automatic electrochemiluminescence immunoassay analyzer (Roche, Germany). All samples were collected at a single time point to minimize temporal variations in acute-phase reactant levels.
Statistical analysis. Data analysis was performed using SPSS 22.0 statistical software. Measurement data that met normal distribution were expressed as mean±standard deviation (x±s). Inter-group comparisons were carried out using one-way analysis of variance (ANOVA) and pairwise comparison using the SNK-q test. Measurement data that deviated from the normal distribution were represented by the median (interquartile range) [M (Q1, Q3)], with inter-group comparison performed using the Kruskal-Wallis H-Test, and pairwise comparison adopted the Mann-Whitney U-test. Counting data were expressed in the form of cases (percentage) [n (%)]; the chi-square test was used for inter-group comparison, and the Bonferroni-corrected chi-square test was used for pairwise comparison. A value of P<0.05 indicated the presence of a statistically significant difference. The computer program MedCalc 15.0 was used for receiver operating characteristic (ROC) curve analysis. Indicators with statistically significant differences in the univariate analysis were incorporated in the forward stepwise conditional Logistic regression analysis to establish a Logistic regression model for predicting NS pathogen types so as to identify the independent, influential factors for determining Gram-positive and Gram-negative bacterial infections.
Results
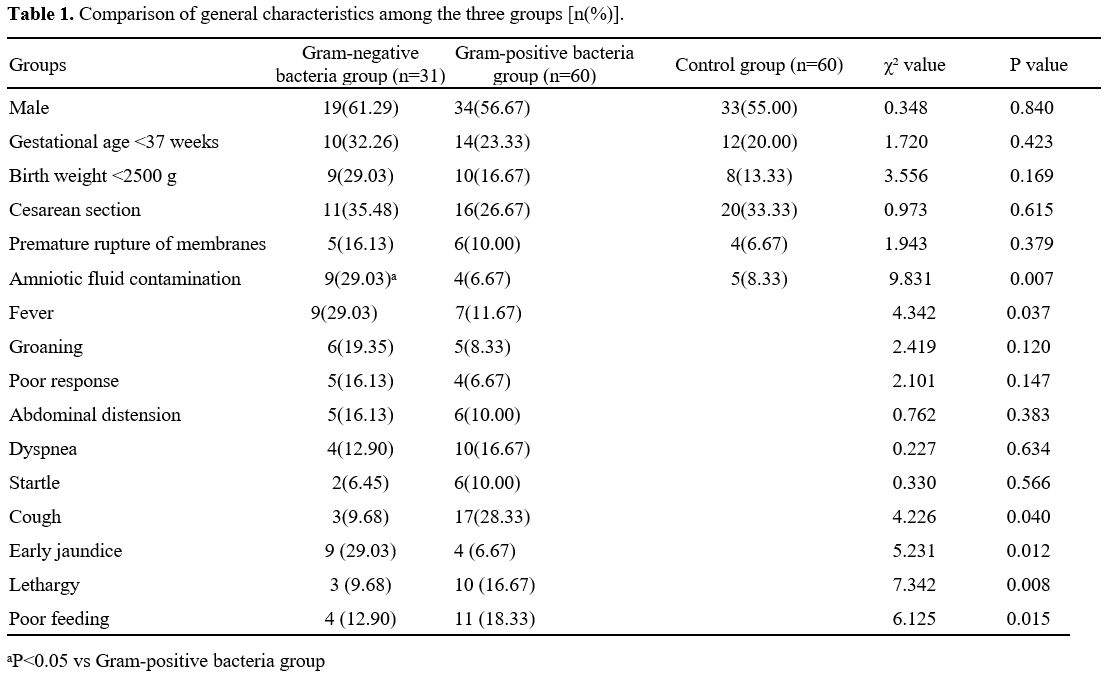
Comparison of general data among groups. Table 1 illustrates that there was no statistically significant difference in general data such as gender, gestational age, birth weight, incidence of premature birth, and incidence of premature rupture of membranes among newborns in the Gram-negative bacteria group, Gram-positive bacteria group, and control group (P>0.05). The incidence of amniotic fluid contamination in Gram-negative and Gram-positive bacteria groups was higher than that in the control group (χ2=9.831, P=0.007).Comparison of symptoms and signs between Gram-negative bacteria group and Gram-positive bacteria group. Table 1 illustrates that the incidence of fever (body temperature ≥ 38. 0 ℃) in the Gram-negative bacteria group surpassed that of the Gram-positive bacteria group (χ2=4.342, P=0.037). Conversely, the incidence of cough was lower in the former group than in the latter group (χ2=4.226, P=0.040). Additionally, early jaundice was more prevalent in the Gram-negative bacteria group (χ2=5.231, P=0.012), while lethargy (χ2=7.342, P=0.008) and poor feeding (χ2=6.125, P=0.015) were more common in the Gram-positive bacteria group. No statistically significant difference was observed in other symptoms and signs (such as groaning and abdominal distension) between the two groups (P>0.05).
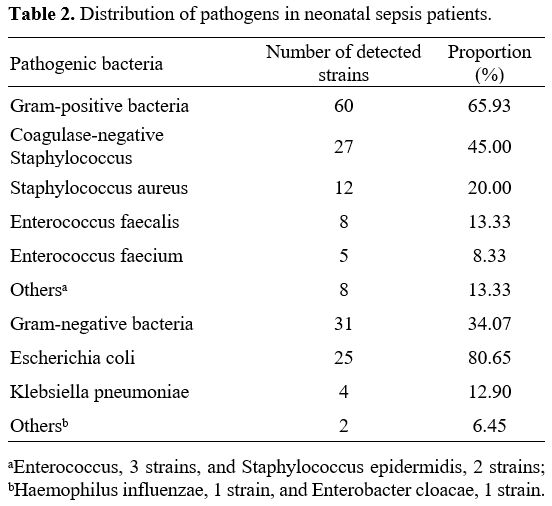
Bacterial distribution in Gram-negative and Gram-positive bacteria groups. Table 2 shows the distribution of pathogens in Gram-negative and Gram-positive bacteria groups. Gram-positive bacteria were mainly coagulase-negative Staphylococcus (27 strains, 45.00%), followed by Staphylococcus aureus (12 strains, 20.00%). At the same time, Gram-negative bacteria were mainly Escherichia coli (25 strains, 80.65%).
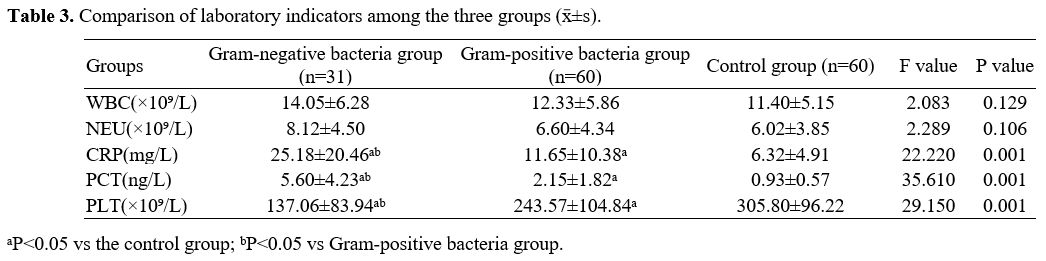
Comparison of laboratory indexes among groups. As presented in Table 3, the levels of CRP and PCT were higher. In contrast, the level of PLT was lower in the Gram-negative bacteria group compared to the Gram-positive bacteria group and control group, with the differences being statistically significant (P<0.05). Moreover, the levels of CRP and PCT were higher, while the level of PLT was lower in the Gram-positive bacteria group than in the control group (P<0.05). Additionally, no statistically significant difference was observed in the comparison of WBC and NEU among groups (P>0.05).
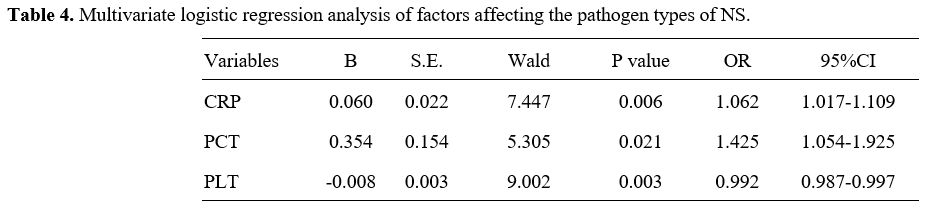
Multivariate Logistic regression analysis on the pathogen types of NS. Indicators with statistically significant differences in the univariate analysis were incorporated into the Logistic regression model for multivariate analysis. The findings presented in Table 4 indicate that CRP, PCT, and PLT serve as independent, influential factors for distinguishing the pathogen types of NS (Gram-positive or Gram-negative).
ROC curve analysis of serum CRP, PCT, and PLT in diagnosing NS caused by Gram-positive and Gram-negative bacterial infections. The area under the ROC curve (AUC) for diagnosing NS attributed to Gram-positive bacterial infections, using Gram-positive bacterial infection as a positive reference standard, were 0.887 for CRP, 0.878 for PCT, and 0.715 for PLT, respectively. As illustrated in Figure 1, the combined AUC of the three indicators was 0.904, with a specificity of 100.00%, both of which were higher than those using separate indicators. The data indicated that the combined detection of serum CRP, PCT, and PLT demonstrated significant diagnostic efficacy in distinguishing between NS caused by Gram-positive and Gram-negative bacterial infections. Table 5 illustrates the ROC curves and AUC of each indicator.
C-reactive protein (CRP), procalcitonin (PCT) and platelets (PLT) were independent influencing factors for differentiating infections caused by the two pathogens (P=0.019, 0.023, 0.030).
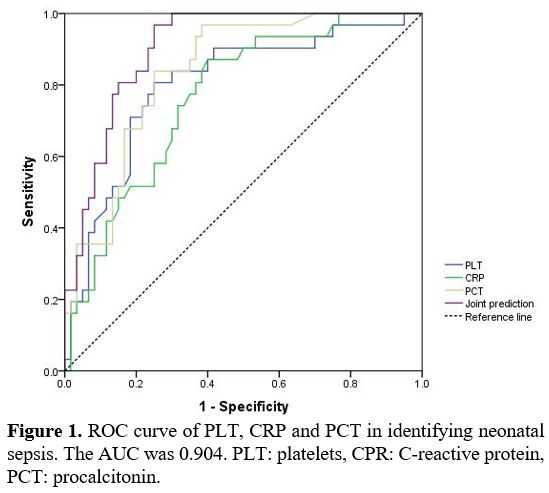 |
Figure 1. ROC curve of PLT, CRP and PCT in identifying neonatal sepsis. The AUC was 0.904. PLT: platelets, CPR: C-reactive protein, PCT: procalcitonin. |
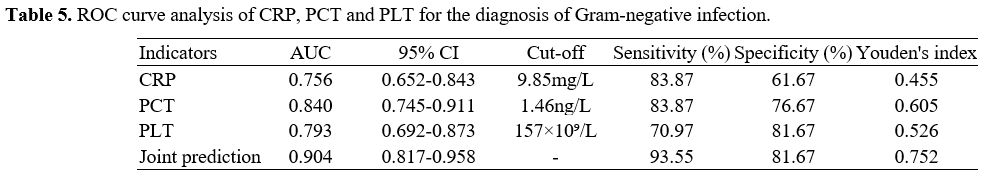 |
Table 5. ROC curve analysis of CRP, PCT and PLT for the diagnosis of Gram-negative infection. |
Discussion
This study found that the proportions of amniotic fluid contamination and fever were higher in pediatric patients with NS. At the same time, the incidence of cough was lower in the Gram-negative bacteria group than those in the Gram-positive bacteria group. Early jaundice was prevalent in the Gram-negative bacteria group, while lethargy and poor feeding were more common in the Gram-positive bacteria group. The incidence of amniotic fluid contamination in Gram-negative and Gram-positive bacteria groups was higher than that in the control group. These findings suggest that newborns with Gram-negative bacterial infections are prone to intrauterine infections and severe systemic infections. Prior research has documented that positive bacterial culture in amniotic fluid was a high-risk factor for early-onset infection in newborns, which can lead to adverse outcomes such as sepsis.[13] Prior research has documented that positive bacterial culture in amniotic fluid was a high-risk factor for early-onset infection in newborns, which can lead to adverse outcomes such as sepsis.[14] Yin et al.[15] also found in their study that amniotic fluid contamination was an independent risk factor for Gram-negative bacterial infection. In general, NS lacks specificity in the clinical manifestations, but it may show varied symptoms and signs under the influence of different pathogens. A foreign study[16] revealed that compared to Gram-positive bacterial infections, Gram-negative bacterial infections might induce symptoms such as fever and respiratory distress more frequently, suggesting a possibly higher severity of Gram-negative bacterial infections.In this study, Gram-positive and Gram-negative bacteria were mainly coagulase-negative Staphylococcus and Escherichia coli. These findings were consistent with those reported in most domestic and foreign research.[17,18] It should be noted that different hospitals and geographical areas may have varied pathogen distribution. For instance, a multi-centre study in China revealed that Group B Streptococcus was the most common type in early-onset NS, while late-onset NS was mainly caused by coagulase-negative Staphylococcus.[19] As a result, heterogeneity in geography, hospitals, and patients should be considered when analyzing and comparing different research results.
Currently, CRP and PCT are the two commonly used auxiliary diagnostic indicators for NS. In this study, newborns with NS showed higher levels of CRP and PCT than those without NS, both of which were also higher in the Gram-negative bacteria group than those in the Gram-positive bacteria group. Therefore, in order to diagnose NS and distinguish between its many pathogen types, CRP and PCT may be valuable. Further multivariate analysis also uncovered that CRP and PCT were independent factors predicting the pathogen types of NS. It has been previously reported that the diagnostic value of CRP for Gram-negative bacterial infections was superior to Gram-positive bacterial infections.[20] In an animal experiment, Gram-positive bacterial and fungal infections on PCT were found to have a lesser induction of PCT production than Gram-negative bacterial lipopolysaccharides.[21] Therefore, it is speculated that patients with Gram-negative bacterial infections may have higher levels of PCT than those with Gram-positive bacterial infections. In contrast, some researchers proposed a limited value of PCT in distinguishing between Gram-positive and Gram-negative bacterial infections.[22]
Furthermore, thrombocytopenia is a common hematological complication of NS, which shows a close relationship with the severity of infection. In this study, it was observed that the level of PLT in the Gram-negative bacteria group was significantly lower than that in the Gram-positive bacteria group and the control group. Meanwhile, PLT was an independent influencing factor of NS pathogen types based on multivariate analysis. Consequently, PLT may benefit the differentiation of NS pathogen types, and Gram-negative bacterial infections may induce a higher risk of thrombocytopenia. Similarly, Saber AM et al.[23] disclosed that thrombocytopenia was significantly associated with Gram-negative bacterial septicemia, and PLT of 50×109/L in the count was an independent predictor. It can be explained that the endotoxin of Gram-negative bacteria can directly inhibit the generation and maturation of bone marrow megakaryocytes, leading to an acceleration in platelet destruction.
As illustrated in Figure 1, the combined diagnostic efficacy of CRP, PCT, and PLT in differentiating NS caused by Gram-positive and Gram-negative bacterial infections was better than that of single indicators. Therefore, in actual clinical practice, the testing of CRP, PCT, and PLT jointly can improve the early detection ability of NS pathogen types, thus providing a potential reference for the selection of antibiotics.
This study also has several limitations, such as the experimental design of a retrospective study, smaller sample size and no long-term follow-up. In our study, all samples were collected within the first 6 hours of admission to minimize temporal variations. However, it is important to consider that even within this timeframe, individual differences in the inflammatory response can lead to variations in CRP and PCT levels. Future studies should standardize sampling protocols and consider the dynamic nature of these biomarkers to refine their diagnostic utility in neonatal sepsis further. Prospective cohort studies based on a larger sample size are also required to further explore the application value of CRP, PCT, and PLT in the early differential diagnosis of NS pathogens.
Conclusions
In conclusion, NS newborns with Gram-positive and Gram-negative bacterial infections show differences in clinical characteristics and laboratory indicators. Newborns with Gram-negative bacterial infections are more prone to developing symptoms such as amniotic fluid contamination and fever (body temperature ≥ 38. 0°C), higher CRP and PCT levels, and lower PLT levels in peripheral blood. The combined use of CRP, PCT, and PLT demonstrates significant diagnostic effectiveness in differentiating the two types of infections, making it a valuable approach in clinical implementation.Ethics approval and consent to participate
The present study was conducted in accordance with the Declaration of Helsinki. This retrospective study has been approved by the Ethics Committee of Bengbu First People’s Hospital.Written informed consent was waived because it only included de-identified health information.
Funding
The study is funded by the Key Project of Natural Science Research in Universities of Anhui Province (No. KJ2021A0761).Author Contributions
Duan YH conceived the study. Liang YB and Chen Y participated in its design and collected the data. Guo P and Chang ZY participated in the data analysis and statistics. All authors helped draft the manuscript. All authors read and approved the final manuscript.References
- Pavlyshyn H, Sarapuk I, Kozak K. The relationship
between neonatal stress in preterm infants and developmental outcomes
at the corrected age of 24-30 months. Front Psychol. 2024;15:1415054.
doi: 10.3389/fpsyg.2024.1415054. https://doi.org/10.3389/fpsyg.2024.1415054 PMid:38840740 PMCid:PMC11150848
- Giannoni
E, Agyeman PKA, Stocker M, et al. Neonatal Sepsis of Early Onset, and
Hospital-Acquired and Community-Acquired Late Onset: A Prospective
Population-Based Cohort Study. J Pediatr. 2018;201:106-114.e4. doi:
10.1016/j.jpeds.2018.05.048. https://doi.org/10.1016/j.jpeds.2018.05.048 PMid:30054165
- Gottschalk
A, Coggins S, Dhudasia MB, et al. Utility of Anaerobic Blood Cultures
in Neonatal Sepsis Evaluation. J Pediatric Infect Dis Soc.
2024:piae056. doi: 10.1093/jpids/piae056. https://doi.org/10.1093/jpids/piae056 PMid:38822536
- Daaboul
D, Osman M, Kassem II, et al. Neonatal sepsis due to NDM-1 and VIM-2
co-producing Pseudomonas aeruginosa in Morocco. J Antimicrob Chemother.
2024:dkae153. doi: 10.1093/jac/dkae153. https://doi.org/10.1093/jac/dkae153 PMid:38804143
- Lungu
N, Popescu DE, Manea AM, et al. Hemoglobin, Ferritin, and Lactate
Dehydrogenase as Predictive Markers for Neonatal Sepsis. J Pers Med.
2024;14(5):476. doi: 10.3390/jpm14050476. https://doi.org/10.3390/jpm14050476 PMid:38793057 PMCid:PMC11122012
- Ruan
L, Chen GY, Liu Z, et al. The combination of procalcitonin and
C-reactive protein or presepsin alone improves the accuracy of
diagnosis of neonatal sepsis: a meta-analysis and systematic review.
Crit Care. 2018;22(1):316. doi: 10.1186/s13054-018-2236-1. https://doi.org/10.1186/s13054-018-2236-1 PMid:30463590 PMCid:PMC6249912
- Vardar
G, Ozek E. Carboxyhemoglobin Levels in Preterm Neonatal Late-Onset
Sepsis: to Predict or not to Predict. Mediterr J Hematol Infect Dis.
2023 Mar 1;15(1):e2023017. doi: 10.4084/MJHID.2023.017. https://doi.org/10.4084/MJHID.2023.017 PMid:36908862 PMCid:PMC10000836
- Eschborn
S, Weitkamp JH. Procalcitonin versus C-reactive protein: review of
kinetics and performance for diagnosis of neonatal sepsis. J Perinatol.
2019;39(7):893-903. doi: 10.1038/s41372-019-0363-4. https://doi.org/10.1038/s41372-019-0363-4 PMid:30926891
- Tzialla
C, Manzoni P, Achille C, et al. New Diagnostic Possibilities for
Neonatal Sepsis. Am J Perinatol. 2018;35(6):575-577. doi:
10.1055/s-0038-1639361. https://doi.org/10.1055/s-0038-1639361 PMid:29695000
- The
Group of Neonatology, Society of Pediatrics, Chinese Medical
Association, Editorial board of Chinese Journal of Pediatrics. Protocol
for Diagnosis and Treatment of Neonatal Septicemia. Chinese Journal of
Pediatrics. 2003;41(12):897-899. doi:
10.3760/j.issn:0578-1310.2003.12.005.
- Faul
F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical
power analysis program for the social, behavioral, and biomedical
sciences. Behav Res Methods. 2007 May;39(2):175-91. doi:
10.3758/bf03193146. https://doi.org/10.3758/BF03193146 PMid:17695343
- National Institute of Child Health and Human Development. Classification of amniotic fluid turbidity. Available at: https://www.nichd.nih.gov/health/topics/amnioticfluidturbidity
- Ogundare
E, Akintayo A, Aladekomo T, et al. Presentation and outcomes of early
and late onset neonatal sepsis in a Nigerian Hospital. Afr Health Sci.
2019;19(3):2390-2399. doi: 10.4314/ahs.v19i3.12. https://doi.org/10.4314/ahs.v19i3.12 PMid:32127809 PMCid:PMC7040286
- James
J, Tewari VV, Jain N. Diagnostic Accuracy of Clinical Tool 'STOPS' and
Serum Procalcitonin for Optimizing Antibiotic Therapy in Neonates Born
at ≥ 28 Weeks of Gestation with Neonatal Sepsis. Mediterr J Hematol
Infect Dis. 2021 Mar 1;13(1):e2021019. doi: 10.4084/MJHID.2021.019. https://doi.org/10.4084/mjhid.2021.019 PMid:33747400 PMCid:PMC7938925
- Yin
H, Wu YZ. Advances in Diagnosis and Antibiotic Application of Neonatal
Early-Onset Sepsis. Advances in Clinical Medicine.
2022;12(6):5212-5221. doi: 10.12677/ACM.2022.126755. https://doi.org/10.12677/ACM.2022.126755
- [Mayor-Lynn
K, Víctor Hugo González-Quintero, O'Sullivan MJ, et al. Comparison of
early-onset neonatal sepsis caused by Escherichia coli and group B
Streptococcus. American Journal of Obstetrics & Gynecology.
2005;192(5):1437-1439. doi: 10.1016/j.ajog.2004.12.031. https://doi.org/10.1016/j.ajog.2004.12.031 PMid:15902130
- Guo
J, Luo Y, Wu Y, et al. Clinical Characteristic and Pathogen Spectrum of
Neonatal Sepsis in Guangzhou City from June 2011 to June 2017. Med Sci
Monit. 2019;25:2296-2304. doi: 10.12659/MSM.912375. https://doi.org/10.12659/MSM.912375 PMid:30924465 PMCid:PMC6451358
- Tsai
MH, Chu SM, Lee CW, et al. Recurrent late-onset sepsis in the neonatal
intensive care unit: incidence, clinical characteristics and risk
factors. Clin Microbiol Infect. 2014;20(11):O928-935. doi:
10.1111/1469-0691.12661. https://doi.org/10.1111/1469-0691.12661 PMid:24796697
- Qiao
YY, Li Y, Li MC, et al. Clinical Features, Distribution of Pathogenic
Bacteria and Drug Resistance in 151 Cases of Neonatal Sepsis. China
Licensed Pharmacist. 2023;20(5):74-79. doi:
10.3969/j.issn.2096-3327.2023.05.011.
- Taşın
C, Coşkun A. The importance of C-reactive protein and procalcitonin in
the diagnosis of chorioamnionitis in the cases with preterm premature
rupture of membranes. Perinatal Journal. 2020;28(3):190-195. doi:
10.2399/prn.20.0283010. https://doi.org/10.2399/prn.20.0283010
- Grati
FR, Ferreira J, Benn P, et al. Outcomes in pregnancies with a confined
placental mosaicism and implications for prenatal screening using
cell-free DNA. Genet Med. 2020;22(2):309-316. doi:
10.1038/s41436-019-0630-y. https://doi.org/10.1038/s41436-019-0630-y PMid:31391534
- Geraerds
AJLM, van Herk W, Stocker M, et al. Cost impact of procalcitonin-guided
decision making on duration of antibiotic therapy for suspected
early-onset sepsis in neonates. Crit Care. 2021;25(1):367. doi:
10.1186/s13054-021-03789-x. https://doi.org/10.1186/s13054-021-03789-x PMid:34670582 PMCid:PMC8529813
- Saber AM, Aziz SP, Almasry AZE, et al. Risk factors for severity of thrombocytopenia in full term infants: a single center study. Ital J Pediatr. 2021;47(1):7. doi: 10.1186/s13052-021-00965-1. https://doi.org/10.1186/s13052-021-00965-1 PMid:33436048 PMCid:PMC7802304