All patients in the age group of 10-70 years who responded to the treatment of their primary hematological cancer during the period between March 2020 and December 2021 and were in active follow-up from 1-Mar-2020 to 31-Mar-2022, with a minimum follow-up of six months after the achievement of complete remission were included in the study. The Institutional Review Board approved the study (DNSH-EC-270622), and written informed consent was obtained from all participants. Data collection, compilation and analysis were carried out after the completion of the observation period. Only those with complete morphological, anatomical, or metabolic remission for acute leukemia and lymphoma and at least a very good partial response for myeloma were included in the study. Any patient with interruption of definitive therapy for more than four weeks or those who succumbed to COVID-19 or relapsed within 30 days of the diagnosis were excluded from the study (2). Relapse or disease progression was diagnosed for individual disease entities based on standard criteria.
All patients included in the study had nasopharyngeal swabs evaluated for SARS-CoV-2 by reverse transcriptase-polymerase chain reaction (RT-PCR) upon developing symptoms suggestive of COVID-19 or following unprotected contact with an individual with COVID-19, either at home or in the hospital. COVID-19 was diagnosed, and its severity was graded according to established criteria.[7]
As part of a simultaneous ongoing project, immunological parameters related to T and NK cell subsets were sequentially monitored by flow cytometry in patients undergoing allogeneic HCT. During the study period, these evaluations were extended to a cohort of non-HCT patients developing COVID-19. Details of the procedure have been described previously.[7-9] The following antibodies were used for phenotypic analysis: CD3(APC-H7, SK-7) CD16 (PE-Cy7, B73.1), CD56 (APC R700, NCAM16.2), CD57 (BV605, NK-1), NKG2A (PE-Cy7, Z199), CD4 (APC-H7), CD8 (Per-CP Cy), CD45RA (FITC), CD45RO (BV605), CD279 (PD-1, BV605) from BD Biosciences, (San Jose, CA) and NKG2C (PE, REA205) from Miltenyi Biotec, Germany. Flow cytometry was performed using 10 colour flow cytometry (BD FACSLyricTM) and the flow cytometry data were analyzed using FlowJo software.
Binary variables were compared between the groups using the chi-square test. Continuous variables were analyzed using the independent sample t-test, which considers Levene’s test for equality of variances and non-parametric tests (Mann-Whitney U test). Probabilities of survival were estimated using the Kaplan-Meier product-limit method. The cumulative incidence rates of relapse were computed by censoring for competing risks using the Fine and Gray method (https://cran.rproject.org/web/packages/cmprsk/index.html). Multivariate analysis was carried out using Cox regression analysis. All analyses were performed using statistical software IBM SPSS Statistics Version 24.0 (Armonk, USA) and GraphPad Prism 9.
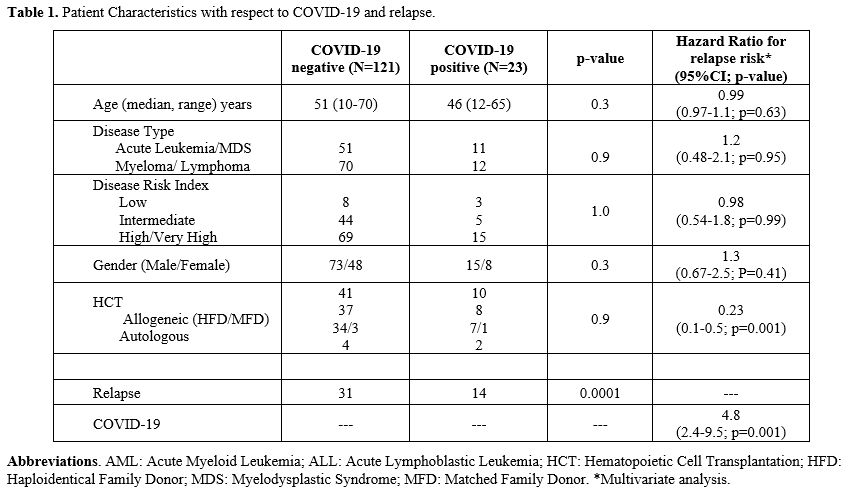
A total of 144 patients were included in this study. Patient and disease characteristics are detailed in Table 1. The median age of the cohort was 50 years (range, 10-70). Acute leukemia or myelodysplastic syndrome, lymphoproliferative disorders, and myeloma accounted for 43%, 39%, and 18%, respectively. All patients had achieved a CR following first-line therapy and were not on active therapy when enrolled in the study. The only exception was those with myeloma, who had achieved at least a VGPR (n=12) and were on oral maintenance therapy with lenalidomide or thalidomide. Based on DRI scoring, 58%, 35%, and 7% of the patients had a high-risk or very-high risk (DRI-high) intermediate risk and low-risk disease (DRI-non-high), respectively. Of the 84 patients in the DRI-high cohort, 45 underwent allogeneic hematopoietic cell transplantation (HCT) (HLA-haploidentical-41; HLA-matched-4), and six underwent an autologous HCT. Minimal residual disease was not considered for analysis as all patients with acute leukemia or MDS were MRD-positive at the time of HCT.
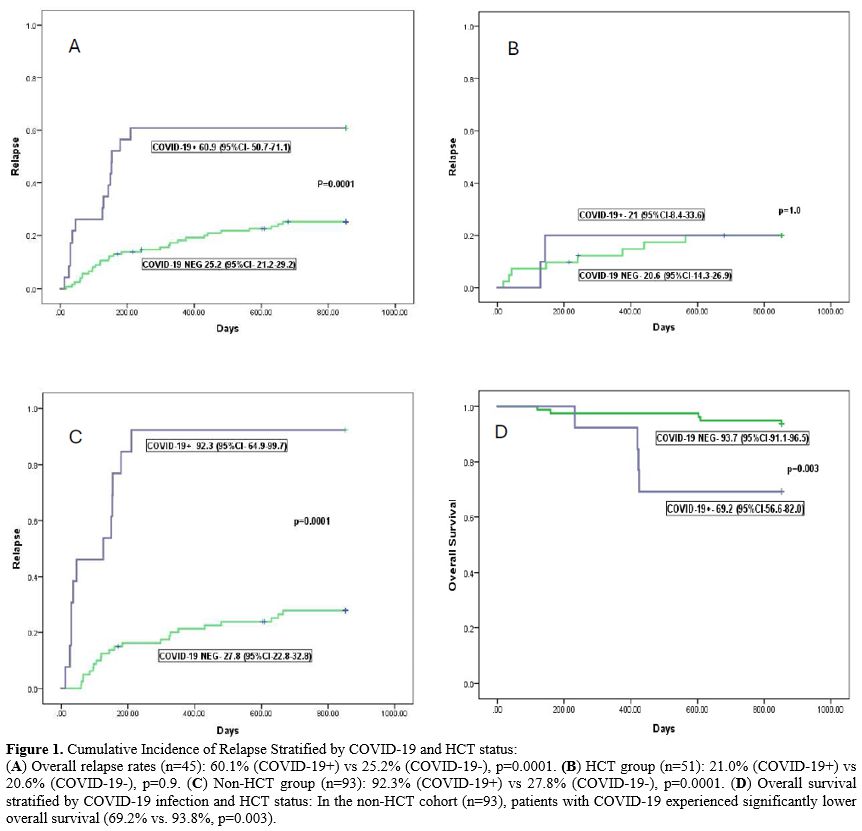
The incidence of COVID-19 was 18.9% (n=23; 95% CI, 15.3-22.5). Moderate to severe COVID-19 was documented in six of them (26%). The median duration of illness was 18 days (range,12-28). The overall incidence of relapse was 30.9% (45 patients). This was 60.9% in those with COVID-19, compared to 25.2% in those without (Figure 1A, HR-3.6[1.9-6.8], p=0.0001). Relapse was not influenced by the severity of COVID-19. There were no deaths directly related to COVID-19.
Relapse tended to occur sooner in patients with COVID-19 (median 127 [range, 32-280] days vs 259 [28-680] days in those without COVID-19, p=0.001). Patients were stratified as DRI-high (including high and very-high DRI) or DRI-non-high (including low and intermediate DRI). The effect of COVID-19 on relapse was more striking in the DRI-high group (64.3% vs 20.1%, p=0.0001), but tended to be higher in the DRI-non-high group as well (55.6% vs 32.5%, p=0.06). There was no influence of disease type on relapse with respect to COVID-19.
There was no difference in the incidence of COVID-19 between HCT and non-HCT patients (10/51 vs 13/93, p=0.5). The incidence of relapse was 22% in the HCT cohort, compared to 36.9% in the non-HCT cohort (p=0.06). There was no difference in relapse in the HCT group, stratified by COVID-19 (20.6% vs 21.0%, p=1.0, Figure 1B). However, in the non-HCT group, the incidence of relapse was 27.8% (22/80) in those without COVID-19, compared to 92.3% (12/13) in the COVID-19 positive group (HR-8.9, 95% CI-4.2-18.9, p=0.0001, Figure 1C). There was no relation between DRI status or disease type and relapse incidence in the HCT group.
On multivariate analysis (Table 1), COVID-19 was the only risk factor for relapse (HR-4.8, 95% CI-2.4-9.5; p=0.0001). On the other hand, HCT was associated with a protective effect against relapse with COVID-19 in the model (HR-0.37, 95% CI-0.2-0.7; p=0.007).
The overall survival in patients with COVID-19 at two years was 82.6%, compared to 94.2% in those without COVID-19 (p=0.05). There was no difference in survival in the HCT cohort stratified by SARS-CoV-2 exposure. However, in the non-HCT cohort, OS was significantly inferior in those with COVID-19 (69.2% VS 93.8%, P=0.003, Figure 1D).
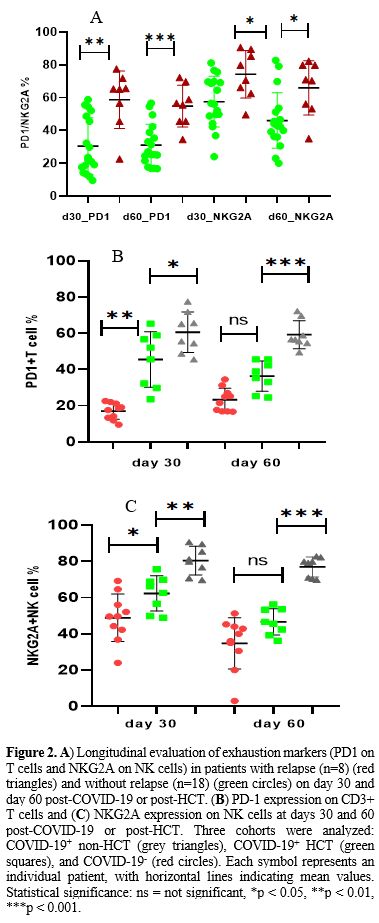
Longitudinal evaluation of PD1 expression on CD3+T cells and NKG2A expression on NK cells was carried out in 16 unselected patients from the COVID-19 exposed cohort at 30-45 days and 60-90 days post-infection; eight patients each from HCT and non-HCT groups. PD expression was also carried out during the same period in 10 COVID-19 non-exposed patients who received an allogeneic HCT at 30 and 60 days post-HCT. Patients who relapsed within 60 days of either diagnosis of COVID-19 or the HCT procedure were not included in the analysis.
Relapse was documented in eight of these 26 patients at a median of 86 days (range 68-152). PD1 (CD279) was significantly upregulated in CD3+T cells in those with relapse at day 30 (65.3±17.5 vs 22.8±17.2 p=0.0001, Figure 2A) and day 60 (54.8±12.8 vs 31±12.8, p=0.001, Figure 2A). A similar trend was noted in NK cells as well (Figure 2A), with regard to expression of the inhibitory NKG2A receptor at day 30 (74.3±14.5 vs 57.5±15.4 p=0.01) and day 60 (66±16.5 vs 46±16.9, p=0.01).
While analyzing the propensity for the higher incidence of relapse in the COVID-19-exposed non-HCT cohort, it was noted that PD1 expression was significantly upregulated in CD3+T cells in the COVID-19+ non-HCT cohort at day 30 (60.6±11.2% vs. 39.8±12.1% in COVID-19+ HCT cohort, p=0.01). PD1 was persistently upregulated in the non-HCT cohort at day +60 compared to the COVID-19+ HCT cohort, where a reduction in PD1 expression was observed (Figure 2B, p=0.002).
On the other hand, the COVID-19 negative HCT cohort showed a lower expression of exhaustion markers on both subsets of T cells at both days +30 and +60. The expression of PD1 was significantly lower compared to the COVID-19-exposed patients from both HCT and non-HCT cohorts at the day 30 assessment. This trend was sustained when compared with the COVID-19+ non-HCT cohort at day 60 (p <0.001) but not for the COVID-19+ HCT cohort (Figure 2B, p=0.2).
The median NKG2A expression at day 30 for the COVID-19 negative HCT cohort was 49.6%, compared to 64.4% (p=0.03) and 80.5% (p<0.0001) in the COVID-19 positive HCT and non-HCT cohorts respectively (Figure 2C). NKG2A expression reduced at day 60 in both HCT cohorts, 38% in the COVID-19 negative (p=0.02) and 46.1% in the COVID-19 positive (p=0.004) cohorts. However, high NKG2A expression was sustained at a median of 79.5% in the non-HCT cohort at 60 days. Thus, the significant difference in NKG2A expression was sustained between the HCT and non-HCT groups (Figure 2C). There was no significant difference in the absolute lymphocyte count, T cell, or NK cell counts amongst patients with and without relapse at days 30 and 60.
The rather strong correlation between COVID-19 and disease relapse across all major types of hematological malignancy was indeed a revelation, as no study has addressed this issue until now. No predilection could be ascertained based on disease type or risk status, which could be due to the small sample size. However, the exclusion of subjects with early COVID-19-related mortality probably helped remove a confounding factor when attributing a cause-effect relation between COVID-19 and relapse.
Two further observations raise the possibility of COVID-19 immunologically impacting the progression of diseases otherwise in clinical CR. The first is the compelling documentation of both T cell and NK cell exhaustion in COVID-19 patients, and the second is the protective effect of an allogeneic HCT in providing protection against sustained immune exhaustion. Exhaustion of T cells has been studied recently in patients with long-COVID-19, and sustained exhaustion has been implicated in protracted illness, which is noted in a subgroup of infected patients.[10-12] We did observe sustained exhaustion in terms of the expression of PD-1 on T cells and NKG2A on NK cells in those who subsequently relapsed. Even though the effect of COVID-19 on immune exhaustion was evident in recipients of allogeneic HCT as well, this effect was significantly less dramatic as well as less sustained than in those who were HCT-naïve. This brings to the fore the possible impact of COVID-19 on an immune system that has sustained the rigor of protracted anticancer therapy versus a rejuvenated immune system derived from a healthy allogeneic graft. The HCT protocol currently employed in our institution has been shown to have a salutary effect on reducing relapse incidence, which was mediated by ANK cells along with the absence of immune exhaustion.[8]
Major limitations of the study are, of course, its retrospective nature and the small sample size. One might argue that the immunological correlates may have been a function of selectivity, and caution must be exercised in the extrapolation of these findings. The use of a wider array of exhaustion markers, such as TIM3, LAG3, or TIGIT, might have helped unravel the correlation better.[11] The same might be said of the selection of NKG2A as a negative regulator of NK cell cytotoxicity. However, COVID-19 has been shown to upregulate NKG2A on NK cells and HLA-E, the cognate ligand on lung epithelial cells, leading to functional exhaustion of NK cells.[13,14] While our group has demonstrated the adverse implications of the upregulation of NKG2A on disease relapse,[8,15] T-cell exhaustion has been shown to be a dominant pathway for immune escape and subsequent relapse.[16] With the above considerations, there might be a case for anti-PD1 therapy in patients at high risk of relapse following COVID-19. While there is no approved therapy for targeting NKG2A upregulated NK cells, our studies on the administration of heat-killed Mycobacterium w (Sepsivac, Cadila, India) as prophylaxis for COVID-19 resulted in downregulation of NKG2A+NK cells.[7]
Retrospective studies that are observational in nature might not provide the perfect answer, yet they might point in the right direction. Thus, the strong correlation between COVID-19 and relapse witnessed in relation to sustained immune exhaustion in the non-HCT cohort vis-à-vis a protective role of allogeneic HCT might prompt more studies with a deeper and better understanding of this phenomenon.bservational in nature might not provide the perfect answer, yet they might point in the right direction. Thus, the strong correlation between COVID-19 and relapse witnessed in relation to sustained immune exhaustion in the non-HCT cohort vis-à-vis a protective role of allogeneic HCT might prompt more studies with a deeper and better understanding of this phenomenon.
References
- T.P. Hanna, G.A. Evans, and C.M. Booth, Cancer, COVID-19 and the
precautionary principle: prioritizing treatment during a global
pandemic. Nat Rev Clin Oncol (2020). https://doi.org/10.1038/s41571-020-0362-6 PMid:32242095 PMCid:PMC7117554
- J.
Jee, M.B. Foote, M. Lumish, A.J. Stonestrom, B. Wills, V. Narendra, V.
Avutu, Y.R. Murciano-Goroff, J.E. Chan, A. Derkach, J. Philip, R.
Belenkaya, M. Kerpelev, M. Maloy, A. Watson, C. Fong, Y. Janjigian,
L.A. Diaz, Jr., K.L. Bolton, and M.S. Pessin, Chemotherapy and COVID-19
Outcomes in Patients With Cancer. J Clin Oncol 38 (2020) 3538-3546. https://doi.org/10.1200/JCO.20.01307 PMid:32795225 PMCid:PMC7571792
- P.
Fedele, V. Sanna, A. Fancellu, A. Marino, N. Calvani, and S. Cinieri,
De-escalating cancer treatments during COVID 19 pandemic: Is metronomic
chemotherapy a reasonable option? Crit Rev Oncol Hematol 157 (2021)
103148. https://doi.org/10.1016/j.critrevonc.2020.103148 PMid:33254036 PMCid:PMC7672334
- H.
Zalpoor, A. Akbari, N. Nayerain Jazi, M. Liaghat, and M. Bakhtiyari,
Possible role of autophagy induced by COVID-19 in cancer progression,
chemo-resistance, and tumor recurrence. Infect Agent Cancer 17 (2022)
38. https://doi.org/10.1186/s13027-022-00450-2 PMid:35850916 PMCid:PMC9289088
- J.
Xie, W. Qi, L. Cao, Y. Tan, J. Huang, X. Gu, B. Chen, P. Shen, Y. Zhao,
Y. Zhang, Q. Zhao, H. Huang, Y. Wang, H. Fang, Z. Jin, H. Li, X. Zhao,
X. Qian, F. Xu, D. Ou, S. Wang, C. Xu, M. Li, Z. Jiang, Y. Wang, X.
Huang, and J. Chen, Predictors for Fear of Cancer Recurrence in Breast
Cancer Patients Referred to Radiation Therapy During the COVID-19
Pandemic: A Multi-Center Cross-Section Survey. Front Oncol 11 (2021)
650766. https://doi.org/10.3389/fonc.2021.650766 PMid:34381703 PMCid:PMC8351463
- Rahimmanesh,
L. Shariati, N. Dana, Y. Esmaeili, G. Vaseghi, and S. Haghjooy
Javanmard, Cancer Occurrence as the Upcoming Complications of COVID-19.
Front Mol Biosci 8 (2021) 813175. https://doi.org/10.3389/fmolb.2021.813175 PMid:35155571 PMCid:PMC8831861
- S.R.
Jaiswal, J. Arunachalam, A. Saifullah, R. Lakhchaura, D. Tailor, A.
Mehta, G. Bhagawati, H. Aiyer, B. Khamar, S.V. Malhotra, and S.
Chakrabarti, Impact of an Immune Modulator Mycobacterium-w on Adaptive
Natural Killer Cells and Protection Against COVID-19. Front Immunol 13
(2022) 887230. https://doi.org/10.3389/fimmu.2022.887230 PMid:35603154 PMCid:PMC9115578
- S.R.
Jaiswal, S. Chakraborty, R. Lakhchaura, P. Shashi, A. Mehta, M. Soni,
and S. Chakrabarti, Early and Sustained Expansion of Adaptive Natural
Killer Cells Following Haploidentical Transplantation and
CTLA4Ig-Primed Donor Lymphocyte Infusions Dissociate
Graft-versus-Leukemia and Graft-versus-Host Effects. Transplant Cell
Ther 27 (2021) 144-151. https://doi.org/10.1016/j.jtct.2020.10.005 PMid:33830023
- S.R.
Jaiswal, J. Arunachalam, A. Bhardwaj, A. Saifullah, R. Lakhchaura, M.
Soni, G. Bhagawati, and S. Chakrabarti, Impact of adaptive natural
killer cells, KLRC2 genotype and cytomegalovirus reactivation on late
mortality in patients with severe COVID-19 lung disease. Clin Transl
Immunology 11 (2022) e1359. https://doi.org/10.1002/cti2.1359 PMid:35035954 PMCid:PMC8752325
- C.
Phetsouphanh, B. Jacka, S. Ballouz, K.J.L. Jackson, D.B. Wilson, B.
Manandhar, V. Klemm, H.X. Tan, A. Wheatley, A. Aggarwal, A. Akerman, V.
Milogiannakis, M. Starr, P. Cunningham, S.G. Turville, S.J. Kent, A.
Byrne, B.J. Brew, D.R. Darley, G.J. Dore, A.D. Kelleher, and G.V.
Matthews, Improvement of immune dysregulation in individuals with long
COVID at 24-months following SARS-CoV-2 infection. Nat Commun 15 (2024)
3315. https://doi.org/10.1038/s41467-024-47720-8 PMid:38632311 PMCid:PMC11024141
- E.
Untersmayr, C. Venter, P. Smith, J. Rohrhofer, C. Ndwandwe, J.
Schwarze, E. Shannon, M. Sokolowska, C. Sadlier, and L. O'Mahony,
Immune Mechanisms Underpinning Long COVID: Collegium Internationale
Allergologicum Update 2024. Int Arch Allergy Immunol 185 (2024)
489-502. https://doi.org/10.1159/000535736 PMid:38253027
- K.
Yin, M.J. Peluso, X. Luo, R. Thomas, M.G. Shin, J. Neidleman, A.
Andrew, K.C. Young, T. Ma, R. Hoh, K. Anglin, B. Huang, U. Argueta, M.
Lopez, D. Valdivieso, K. Asare, T.M. Deveau, S.E. Munter, R. Ibrahim,
L. Standker, S. Lu, S.A. Goldberg, S.A. Lee, K.L. Lynch, J.D. Kelly,
J.N. Martin, J. Munch, S.G. Deeks, T.J. Henrich, and N.R. Roan, Long
COVID manifests with T cell dysregulation, inflammation and an
uncoordinated adaptive immune response to SARS-CoV-2. Nat Immunol 25
(2024) 218-225. https://doi.org/10.1038/s41590-023-01724-6 PMid:38212464 PMCid:PMC10834368
- D.
Bortolotti, V. Gentili, S. Rizzo, A. Rotola, and R. Rizzo, SARS-CoV-2
Spike 1 Protein Controls Natural Killer Cell Activation via the
HLA-E/NKG2A Pathway. Cells 9 (2020). https://doi.org/10.21203/rs.3.rs-31860/v1
- L.
Antonioli, M. Fornai, C. Pellegrini, and C. Blandizzi, NKG2A and
COVID-19: another brick in the wall. Cellular & Molecular
Immunology (2020). https://doi.org/10.1038/s41423-020-0450-7 PMid:32382127 PMCid:PMC7203720
- S.R. Jaiswal, P. Bhakuni, G. Bhagawati, H.M. Aiyer, M. Soni, N. Sharma, R.R. Jaiswal, A. Chakrabarti, and S. Chakrabarti, CTLA4Ig-primed donor lymphocyte infusions following haploidentical transplantation improve outcome with a distinct pattern of early immune reconstitution as compared to conventional donor lymphocyte infusions in advanced hematological malignancies. Bone Marrow Transplant 56 (2021) 185-194. https://doi.org/10.1038/s41409-020-01002-1 PMid:32704091
- R.
Zeiser, and L. Vago, Mechanisms of immune escape after allogeneic
hematopoietic cell transplantation. Blood 133 (2019) 1290-1297. https://doi.org/10.1182/blood-2018-10-846824 PMid:30578254