Acute lymphoblastic leukemia (ALL) is the most common malignancy in childhood.[5] Negative consequences of chemotherapy protocols on the lymphocyte pool impair the immune response to both de novo and memory antigens. Consequently, a decrease in vaccine response occurs after the end of treatment. Immune deficiencies and dysregulation vary based on patient age and chemotherapy intensity.[6] Evidence suggests that current protocols for ALL result in significant losses of humoral immunity against viral vaccine antigens, particularly in young infants. Although patients may become more susceptible to infections due to the decrease or loss of antibodies, revaccination at the end of treatment is recommended as essential for some children.[1] While guidelines exist for the revaccination of patients undergoing hematopoietic stem cell transplantation (HSCT), there is no consensus on post-chemotherapy vaccination for pediatric acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).
This study aimed to investigate the persistence of childhood vaccine immunity following leukemia treatment, emphasizing the critical need for additional vaccine doses.
Methods
Study design. This retrospective, cross-sectional study included 62 patients aged 1-17 years who were followed up for ALL in the Ankara Child Health and Diseases Hematology Oncology Training and Research Hospital, Division of Pediatric Hematology, between January 2013 and June 2016.Ethics statement. The study protocol was approved by the Institutional Review Board (2019-007). Informed consent was obtained from the participants or their parents.
Study population and definitions. The study included patients who had been vaccinated according to the national childhood immunization program before the diagnosis of ALL and had completed their treatment. Patients who voluntarily left the follow-up, experienced a relapse, or were treated with hematopoietic stem cell transplantation (HSCT) were excluded. After a comprehensive analysis of 116 patient records, 62 met the inclusion criteria. We recorded gender, age at diagnosis, leukemia type, and leukemia risk group. Vaccination histories for hepatitis A, hepatitis B, varicella, measles, rubella, and mumps were documented. Treatment was administered in accordance with the ALL IC-BFM 2009 protocol.[7] Patients in the standard and intermediate-risk groups were evaluated as a single cohort.
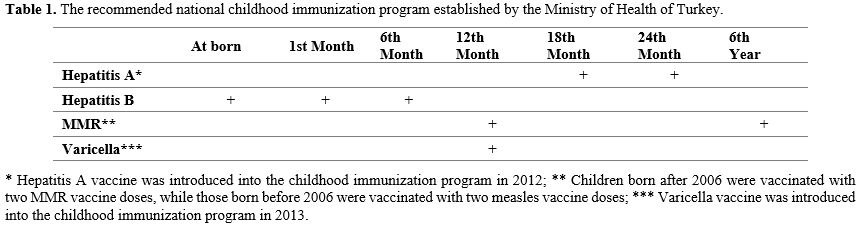
Vaccine Schedule in Turkey. Patients were vaccinated according to the national childhood immunization program of the Turkish Ministry of Health before the diagnosis of ALL (Table 1). In our clinical practice, leukemia patients were generally not vaccinated during chemotherapy, except for the hepatitis B vaccine. Unlike other vaccines, patients identified as hepatitis B seronegative during chemotherapy were immunized with the hepatitis B vaccine at 0, 1, and 6 months while receiving treatment. Vaccine serologies were analyzed at the end of maintenance chemotherapy. Seronegative patients for hepatitis A received two doses of the hepatitis A vaccine after three months following the completion of the chemotherapy protocol. Patients with negative varicella serology were immunized with a single dose of the varicella vaccine one year after completing chemotherapy. MMR-negative patients were vaccinated with a single dose of the MMR vaccine at least six months after the end of treatment.
 |
|
Laboratory Parameters. The cut-off levels for seropositivity were determined using the manufacturer's laboratory test references. Serum anti-HB levels were measured by enzyme-linked immunosorbent assay (ELISA- Architect i2000 SR, Abbott, Turkey), and anti-HB levels ≥10 mIU/mL were considered seropositive. HAV IgG serology was studied using the Chemiluminescent Microparticle Immunological (CMIA- Architect i2000 SR, Abbott, Turkey) test method. Patients with sample signal/cut-off (S/CO) <1 were considered seronegative, and those with S/CO ≥1 were considered seropositive. The ELISA TRITURUS® test kit from VIRCELL, Turkey, was used to detect varicella, measles, and mumps. A value of 0-9/index was considered seronegative, 9-11/index was considered intermediate, and 11-1000/index was considered seropositive. Patients with intermediate serology values for measles, mumps, and varicella were considered seronegative and were vaccinated accordingly. Rubella serology was performed using ELISA (Architect i2000 SR, Abbott, Turkey); values <9U/mL were considered seronegative, and values ≥9U/mL were considered seropositive.
Statically Analyses. All statistical analyses were conducted using SPSS software (version 22.0; SPSS Inc.). Descriptive statistics for categorical data are reported as counts and percentages, while continuous variables are presented as mean, standard deviation, minimum, and maximum values (min-max). The Mann-Whitney U test was employed to compare continuous variables between the two groups. The Chi-square test was used to evaluate differences between categorical variables. Relationships were assessed at a 95% confidence interval, and a p-value of <0.05 was considered statistically significant.
Results
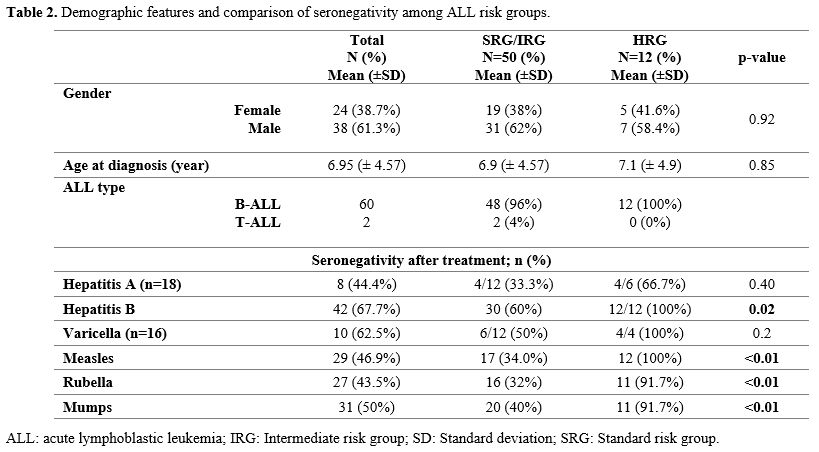
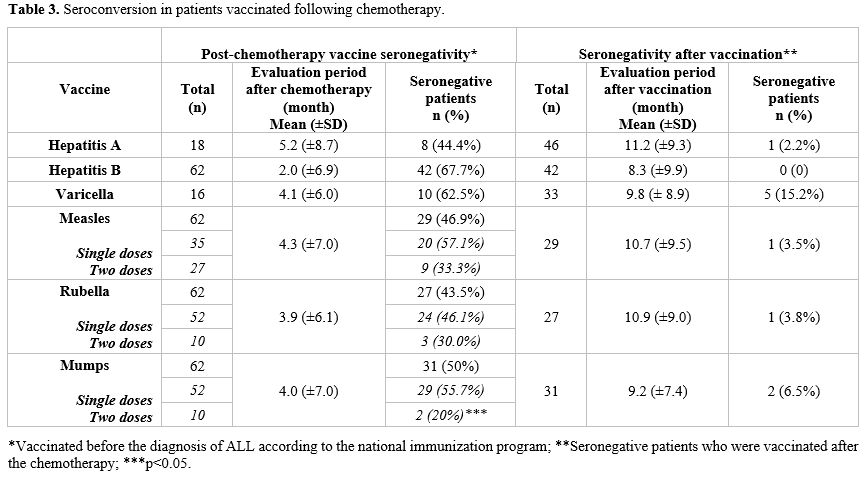
Sixty patients were diagnosed with pre-B-cell ALL and two with T-cell ALL. Of the patients, 24 (38.7%) were female. The mean age at diagnosis was 6.95 ± 4.57 years. Fifty patients (80.6%) were classified in the standard- and intermediate-risk groups (SRG-IRG), while 12 patients (19.4%) were categorized as high-risk (HRG) (Table 2).Vaccine status before chemotherapy. Before diagnosis, all patients had been vaccinated against hepatitis B and MMR. Among those immunized, 52 patients received a single rubella and mumps vaccine, 35 patients received a single measles vaccine, and all the remaining patients received two doses. Furthermore, 18 patients were vaccinated for hepatitis A, and 16 were vaccinated for varicella prior to their leukemia diagnosis (Table 3).
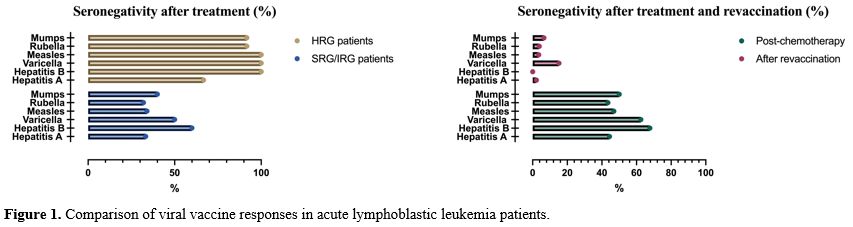
Seronegativity status to vaccines after chemotherapy. At the end of chemotherapy, seronegativity for hepatitis A, hepatitis B, varicella, measles, rubella, and mumps was observed in 8/18 (44.4%), 42/62 (67.7%), 10/16 (62.5%), 29/62 (46.9%), 27/62 (43.5%), and 31/62 (50%) patients, respectively (Figure 1) (Table 2). In HRG ALL patients, seronegativity for hepatitis A (66.7%), hepatitis B (100%), varicella (100%), measles (100%), rubella (91.7%), and mumps (91.7%) were higher than IRG-SRG ALL patients. Seronegativity for hepatitis B, measles, rubella, and mumps was statistically significantly higher in the HRG patients (p <0.05) (Figure 1) (Table 2). The assessment of antibody response at the end of treatment revealed the following mean intervals: 5.2 (±8.7) months for hepatitis A, 2.0 (±6.9) months for hepatitis B, 4.1 (±6.0) months for varicella, 4.3 (±7.0) months for measles, 3.9 (±6.1) months for rubella, and 4.0 (±7.0) months for mumps (Table 3).
The evaluation of patients regarding MMR vaccination doses revealed no significant difference in seroconversion between those receiving a single dose and those receiving two doses for measles and rubella (p > 0.05). However, negative serology for the mumps vaccine was significantly higher among patients who received a single dose (p < 0.05) (Table 3).
All patients have been vaccinated against hepatitis B prior to the diagnosis of leukemia. However, 29 patients whose anti-HB antibodies were tested to evaluate the etiology of hypertransaminasemia during chemotherapy were found to be seronegative. These patients were revaccinated in the different phases of chemotherapy with three doses. At the end of treatment, 16 of the 29 patients vaccinated during chemotherapy (55.1%) remained seronegative. However, of the 33 patients who were not vaccinated during chemotherapy, 26 (78.7%) remained seronegative. No significant difference in seronegativity was observed between the two groups (p>0.05).
Vaccine responses of seronegative patients vaccinated after chemotherapy. Among the seronegative patients, 46 received the hepatitis A vaccine, 42 received the hepatitis B vaccine, 33 received the varicella vaccine, 29 received the measles vaccine, 27 received the rubella vaccine, and 31 received the mumps vaccine. The number of cases who remained seronegative after revaccination was five (15.2%) for varicella, one (2.2%) for hepatitis A, one (3.5%) for measles, one (3.8%) for rubella, and two (6.5%) for mumps (Figure 1) (Table 3).
Discussion
Recent advances in treatment and supportive care have significantly improved survival rates for children with leukemia. However, it is crucial to closely monitor for potential late complications that may develop following chemotherapy. Monitoring the recovery of the immune response is an important consideration among these factors. Patients demonstrate reduced specific vaccine antibodies following chemotherapy protocols. Re-immunization against life-threatening, vaccine-preventable infectious diseases is a critical component of supportive care for pediatric leukemia patients. Although the impact of standard chemotherapy on immunity to these diseases is receiving increasing attention, there are only a limited number of guidelines addressing the revaccination of children receiving standard antileukemia chemotherapy.[8] While the Infectious Diseases Society of America (IDSA) advises routine revaccination with a single dose of each vaccine, the necessity of this practice remains uncertain.[9] The European Conference on Infections in Leukaemia (ECIL) group recommends that for children with acute leukemia, during induction and re-induction chemotherapy, only HBV vaccination should be given in high-risk HBV settings. Varicella vaccination should be given to seronegative children, preferably 3-6 months after chemotherapy completion. After chemotherapy, a booster dose of all vaccines is recommended for those previously vaccinated, and a full vaccination schedule according to age and national guidelines for those who have never been vaccinated.[4]Our study reveals that approximately half of leukemia patients lost vaccine-derived immunity by the end of chemotherapy. Seronegativity for vaccine-related immunity against hepatitis A, hepatitis B, varicella, measles, rubella, and mumps was observed in 44.4%, 67.7%, 62.5%, 46.9%, 43.5%, and 50% of patients, respectively. Seronegativity was found to be significantly high for all viruses evaluated in terms of vaccine response in HRG ALL patients. The existing literature reveals that seroconversion rates for hepatitis B range from 9% to 75%, and the seronegativity rates for varicella vaccine antibodies were reported as 14% to 48%.[1,6,10-14] The seroconversion rates at the end of chemotherapy for measles, rubella, and mumps have been reported to range 39-75,4% for measles, 46-76% for rubella, and 50-71,9% for mumps.[1,2,6,15-17] The variation in the response to the hepatitis A vaccine has not been extensively investigated, and antibody loss against hepatitis A has been reported to be between 54% and 83%.[1,2] Factors affecting antibody levels after chemotherapy include the primary diagnosis, intensity of the chemotherapy protocol, local epidemiology of infectious diseases, immunization schedule, definitions of protective antibody levels, and the types of immunization products administered.
Hepatitis B is a significant concern for patients undergoing chemotherapy in regions where the virus is prevalent. Studies indicate that the prevalence of hepatitis B infection among patients with ALL undergoing chemotherapy can be very high, ranging from 16% to 45%.[18] This datum highlights the importance of screening and vaccination strategies to reduce the risk of infection during treatment. The vaccine antibody response may diminish toward the end of chemotherapy in leukemia patients; however, the timing of this reduction remains uncertain. Several studies have evaluated the seronegativity of patients who received the hepatitis B vaccine during chemotherapy and found that 56% to 81% of patients were still seronegative at the end of chemotherapy protocol.[10,19,20] Yıldırım et al. reported that 74% of seropositive patients who were not vaccinated at the start of chemotherapy maintained their seropositivity.[10] The difference may be due to factors such as malignancy type (ALL vs. non-ALL), vaccine type (recombinant vs. plasma-derived), number of doses (3–5), dose strength (single vs. double), vaccination timing (induction vs. consolidation phase), timing of titer. Our study found no significant difference in the rate of seronegativity at the end of chemotherapy between patients who were seronegative and were vaccinated for hepatitis B during treatment and those who were not vaccinated. Although variations in prevalence and incidence rates may be observed in countries with high levels of immigration like ours, the prevalence of HBsAg positivity in our country is reported to be above 3%.[21] None of our patients developed hepatitis B infection despite declining protective antibody titer at the end of chemotherapy. It is considered that this may be due to the fact that all blood products transfused in our country can only be used after undergoing nucleic acid amplification (NAT) testing. Nevertheless, it is recommended to vaccinate during the induction phase, as it shows higher seroprotection rates compared to the maintenance period, followed by revaccination post-chemotherapy, particularly in countries with a high prevalence of hepatitis B infection.[22]
Age and vaccine dose are also risk factors for losing protective antibody levels. Some studies involving pediatric ALL patients have demonstrated that the vaccine antibody response is lower in younger age groups.[1,2,6,10,17] In our study, the antibody response to the mumps vaccine was significantly higher in patients who received two doses. This enhanced response may be attributed to receiving the MMR vaccine after the age of seven or to the number of vaccine doses administered. In healthy children, the mean antibody titer against the varicella vaccine increased during the first six years after vaccination.[23]
Vaccine antibody loss is higher in the high-risk leukemia group patients compared to the intermediate and standard-risk groups. Like our study, there are studies in the literature in which vaccine antibody loss was higher in the HRG-ALL compared to the IRG-SRG.[1,2,6] However, no difference in vaccine antibody responses between the risk groups was also reported.[8,15] High-risk leukemia patients received more aggressive chemotherapy; it is assumed that the immunosuppression would be much more profound.
Our study found that the seropositivity rate after revaccination and the response levels to each booster dose varied significantly among patients, ranging from 85% to 100%, which is comparable to rates observed in healthy children post-vaccination.[24-26] Anafy et al. reported that seronegative patients after chemotherapy had vaccine response rates between 68% and 100% for hepatitis A, hepatitis B, measles, and varicella.[1] Patel et al. noted a 94% response rate for measles, while Garcia et al. found response rates of 71% to 96% for MMR and varicella.[14,27] In contrast, Fouda et al. observed post-vaccination response rates of 57.1% for measles, 78.6% for rubella, and 87.5% for mumps.[15] The observed differences in seroconversion rates may be attributed to variations in the definitions of protective antibody titers and the number of MMR vaccine doses administered prior to diagnosis.
Vaccines may exhibit reduced efficacy during periods of altered immunocompetence. In such circumstances, the primary concern lies with their effectiveness rather than safety. Moreover, if a non-live vaccine is administered during a phase of chemotherapy, it may be necessary to repeat the vaccination once immune competence has been restored.[9]
Our study has several limitations. It is retrospective in design, which prevents us from comparing antibody titers before and after chemotherapy for each patient. The limited number of high-risk patients diminished the statistical power of risk assessment.
Conclusions
Our study indicates that ALL patients, particularly those in the HRG, had high seronegativity of hepatitis A, hepatitis B, MMR, and varicella at the end of treatment. Therefore, we recommend testing for vaccine-preventable disease immunity after chemotherapy and revaccination.Ethics approval
This study was approved by the University of Health Sciences, Ankara Child Health and Diseases Hematology Oncology Training and Research Hospital Ethics Committee (2019-007).Informed consent
All the participants in this study provided written informed consent, and no participant information was obtained.Author Contributions
EKK had full access to all data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. EKK and HNY designed the study and wrote the manuscript. AKY, AKU, and BCC provided conceptual advice. All authors read and approved the final manuscript.References
- Anafy A, Gilad G, Michaan N, et al. Revaccination
of children with acute lymphoblastic leukemia following completion of
chemotherapy. Pediatr Blood Cancer 2023; 70: e30321. 2023/04/11. DOI:
10.1002/pbc.30321. https://doi.org/10.1002/pbc.30321 PMid:37036274
- Toret
E, Yel SE, Suman M, et al. Immunization status and re-immunization of
childhood acute lymphoblastic leukemia survivors. Hum Vaccin Immunother
2021; 17: 1132-1135. 2020/09/04. DOI: 10.1080/21645515.2020.1802975. https://doi.org/10.1080/21645515.2020.1802975 PMid:32882157 PMCid:PMC8018341
- Perkins
JL, Harris A and Pozos TC. Immune Dysfunction After Completion of
Childhood Leukemia Therapy. J Pediatr Hematol Oncol 2017; 39: 1-5.
2016/11/08. DOI: 10.1097/mph.0000000000000697. https://doi.org/10.1097/MPH.0000000000000697 PMid:27820131
- Mikulska
M, Cesaro S, de Lavallade H, et al. Vaccination of patients with
haematological malignancies who did not have transplantations:
guidelines from the 2017 European Conference on Infections in Leukaemia
(ECIL 7). Lancet Infect Dis 2019; 19: e188-e199. 2019/02/13. DOI:
10.1016/s1473-3099(18)30601-7. https://doi.org/10.1016/S1473-3099(18)30601-7 PMid:30744964
- Pillai
PM and Carroll WL. Chapter 18 - Acute lymphoblastic leukemia. In: Fish
JD, Lipton JM and Lanzkowsky P (eds) Lanzkowsky's Manual of Pediatric
Hematology and Oncology (Seventh Edition). Academic Press, 2022,
pp.413-438. https://doi.org/10.1016/B978-0-12-821671-2.00004-0
- Bochennek
K, Allwinn R, Langer R, et al. Differential loss of humoral immunity
against measles, mumps, rubella and varicella-zoster virus in children
treated for cancer. Vaccine 2014; 32: 3357-3361. 2014/05/06. DOI:
10.1016/j.vaccine.2014.04.042. https://doi.org/10.1016/j.vaccine.2014.04.042 PMid:24793952
- ALL-IC
BFM-2009. A Randomized Trial of the I-BFM-SG for the Management of
Childhood Non-B Acute Lymphoblastic Leukemia. Final Version of Therapy
Protocol from August-14-2009. 2009.
- Nilsson
A, De Milito A, Engström P, et al. Current chemotherapy protocols for
childhood acute lymphoblastic leukemia induce loss of humoral immunity
to viral vaccination antigens. Pediatrics 2002; 109: e91. 2002/06/04.
DOI: 10.1542/peds.109.6.e91. https://doi.org/10.1542/peds.109.6.e91 PMid:12042585
- Rubin
LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline
for vaccination of the immunocompromised host. Clin Infect Dis 2014;
58: 309-318. 2014/01/15. DOI: 10.1093/cid/cit816. https://doi.org/10.1093/cid/cit816 PMid:24421306
- Keskin
Yildirim Z and Buyukavci M. Assessment of Humoral Immunity to Hepatitis
B, Measles, Rubella, and Mumps in Children After Chemotherapy. J
Pediatr Hematol Oncol 2018; 40: e99-e102. 2018/01/09. DOI:
10.1097/mph.0000000000001072. https://doi.org/10.1097/MPH.0000000000001072 PMid:29309372
- Cesaro
S, Giacchino M, Fioredda F, et al. Guidelines on vaccinations in
paediatric haematology and oncology patients. Biomed Res Int 2014;
2014: 707691. 2014/05/29. DOI: 10.1155/2014/707691. https://doi.org/10.1155/2014/707691 PMid:24868544 PMCid:PMC4020520
- Wang
L, Hu H, Zhang R, et al. Changes in the hepatitis B surface antibody in
childhood acute lymphocytic leukaemia survivors after treatment with
the CCLG-ALL 2008 protocol. Clin Exp Immunol 2021; 203: 80-86.
2020/09/17. DOI: 10.1111/cei.13513. https://doi.org/10.1111/cei.13513 PMid:32936935 PMCid:PMC7744497
- Patel
SR, Bate J, Maple PA, et al. Varicella zoster immune status in children
treated for acute leukemia. Pediatr Blood Cancer 2014; 61: 2077-2079.
2014/05/03. DOI: 10.1002/pbc.25086. https://doi.org/10.1002/pbc.25086 PMid:24789692
- de
de la Fuente Garcia I, Coïc L, Leclerc JM, et al. Protection against
vaccine preventable diseases in children treated for acute
lymphoblastic leukemia. Pediatr Blood Cancer 2017; 64: 315-320.
2016/10/09. DOI: 10.1002/pbc.26187. https://doi.org/10.1002/pbc.26187 PMid:27718310
- Fouda
AE, Kandil SM, Boujettif F, et al. Humoral immune response of childhood
acute lymphoblastic leukemia survivors against the measles, mumps, and
rubella vaccination. Hematology 2018; 23: 590-595. 2018/04/05. DOI:
10.1080/10245332.2018.1460035. https://doi.org/10.1080/10245332.2018.1460035 PMid:29614919
- Fayea
NY, Fouda AE and Kandil SM. Immunization status in childhood cancer
survivors: A hidden risk which could be prevented. Pediatr Neonatol
2017; 58: 541-545. 2016/08/21. DOI: 10.1016/j.pedneo.2016.04.003. https://doi.org/10.1016/j.pedneo.2016.04.003 PMid:27543381
- Garonzi
C, Balter R, Tridello G, et al. The Impact of Chemotherapy after
Pediatric Malignancy on Humoral Immunity to Vaccine-Preventable
Diseases. Mediterr J Hematol Infect Dis 2020; 12: e2020014. 2020/03/18.
DOI: 10.4084/mjhid.2020.014. https://doi.org/10.4084/mjhid.2020.014 PMid:32180909 PMCid:PMC7059740
- Guruprasad
B, Kavitha S, Aruna Kumari BS, et al. Risk of hepatitis B infection in
pediatric acute lymphoblastic leukemia in a tertiary care center from
South India. Pediatr Blood Cancer 2014; 61: 1616-1619. 2014/05/07. DOI:
10.1002/pbc.25065. https://doi.org/10.1002/pbc.25065 PMid:24798418
- Nayak
S, Gupta S, Kumar P, et al. A Study of Immunogenicity of Intensified
Hepatitis B Vaccination in Children Being Treated for Acute
Lymphoblastic Leukemia. Indian J Pediatr 2020; 87: 217-218. 2020/01/12.
DOI: 10.1007/s12098-019-03147-4. https://doi.org/10.1007/s12098-019-03147-4 PMid:31925714
- Yetgin
S, Tavil B, Aytac S, et al. Unexpected protection from infection by two
booster hepatitis B virus vaccination in children with acute
lymphoblastic leukemia. Leuk Res 2007; 31: 493-496. 2006/08/26. DOI:
10.1016/j.leukres.2006.06.024. https://doi.org/10.1016/j.leukres.2006.06.024 PMid:16930691
- Tozun
N, Ozdogan O, Cakaloglu Y, et al. Seroprevalence of hepatitis B and C
virus infections and risk factors in Turkey: a fieldwork TURHEP study.
Clin Microbiol Infect 2015; 21: 1020-1026. 2015/07/15. DOI:
10.1016/j.cmi.2015.06.028. https://doi.org/10.1016/j.cmi.2015.06.028 PMid:26163105
- Peringeth
G, Mohan P, Dhodapkar RM, et al. Comparison of efficacy of hepatitis B
vaccination during induction versus maintenance phase of chemotherapy
in acute lymphoblastic leukemia: A nonrandomized clinical trial.
International Journal of Advanced Medical and Health Research 2019; 6:
68 - 73. https://doi.org/10.4103/IJAMR.IJAMR_113_19
- Vessey
SJ, Chan CY, Kuter BJ, et al. Childhood vaccination against varicella:
persistence of antibody, duration of protection, and vaccine efficacy.
J Pediatr 2001; 139: 297-304. 2001/08/07. DOI: 10.1067/mpd.2001.116051.
https://doi.org/10.1067/mpd.2001.116051 PMid:11487760
- Elbahrawy
A, Atalla H, Alboraie M, et al. Recent Advances in Protective Vaccines
against Hepatitis Viruses: A Narrative Review. Viruses 2023; 15
2023/01/22. DOI: 10.3390/v15010214. https://doi.org/10.3390/v15010214 PMid:36680254 PMCid:PMC9862019
- McLean
HQ, Fiebelkorn AP, Temte JL, et al. Prevention of measles, rubella,
congenital rubella syndrome, and mumps, 2013: summary recommendations
of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm
Rep 2013; 62: 1-34. 2013/06/14.
- Marin
M, Güris D, Chaves SS, et al. Prevention of varicella: recommendations
of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm
Rep 2007; 56: 1-40. 2007/06/23.
- Patel SR, Ortín M, Cohen BJ, et al. Revaccination of children after completion of standard chemotherapy for acute leukemia. Clin Infect Dis 2007; 44: 635-642. 2007/02/06. DOI: 10.1086/511636. https://doi.org/10.1086/511636 PMid:17278052