Despite ATRA and ATO may induce morphological dysplasia during treatment (https://imagebank.hematology.org/image/60848/arsenicinduced-dysplasia-in-apl), to our knowledge studies on morphologic changes in bone marrow (BM) progenitor cells during follow-up of ATRA-ATO therapy, in patients achieving morphological and molecular remission, have not been reported. Therefore, taking into account the potential dysplastic effects of ATO treatment,[5] we systematically assessed the rate of bone marrow (BM) dysplastic changes by leveraging the BM slide archives of APL patients treated at Tor Vergata University Hospital, Rome, Italy.
Thus, we gathered BM slides of patients treated with ATRA-ATO at our Center and evaluated the dysplastic features or unexpected morphologic changes at different time points after ATO treatment. Longitudinal BM evaluations were used to verify the persistence of morphological alterations. As a control, we analyzed the bone marrow smears of patients treated with the AIDA chemotherapy combination.[6]
This study enrolled 11 adult patients diagnosed with APL from October 2010 to May 2020, for whom longitudinal BM slides were available. BM evaluations were performed 3 months after the end of consolidation for both protocols and then 12 months (median, range: 11-15 months) after consolidation or maintenance for ATRA/ATO and AIDA regimens, respectively. The morphological BM revision was independently performed by two experienced morphologists (E.S. and S.F.). The evaluation of dysplastic features was performed according to established criteria.[7,8] The grade of dysplasia was defined as follows: absent (grade 0): no dysplastic features; mild (grade 1): dysplastic features present in <10% of one cell lineage; moderate (grade 2): dysplastic features in 10-20% of one lineage; severe (grade 3): dysplastic features in > 20% of a single cell lineage. To evaluate dysplasia (cut-off ≥10% per cell lineage), at least 100 granulocytes, 100 erythroid precursors, and 30 megakaryocytes were evaluated. Unconventional features, such as the presence of eosinophils, mastocytes, promonocytes, and blasts, were also considered.
For statistical analysis, continuous variables from patients treated with ATRA-ATO versus AIDA were compared using the Student t-test or Mann-Whitney U test, as appropriate. Continuous variables observed at different time points (pairing post-consolidation and follow-up samples) following ATRA-ATO were compared using the Student t-test or Wilcoxon test, as appropriate, after checking for a normal distribution of variables.
Overall, patients’ median age at APL onset was 52 years (range: 36-71), with a 1.2 male-to-female ratio.
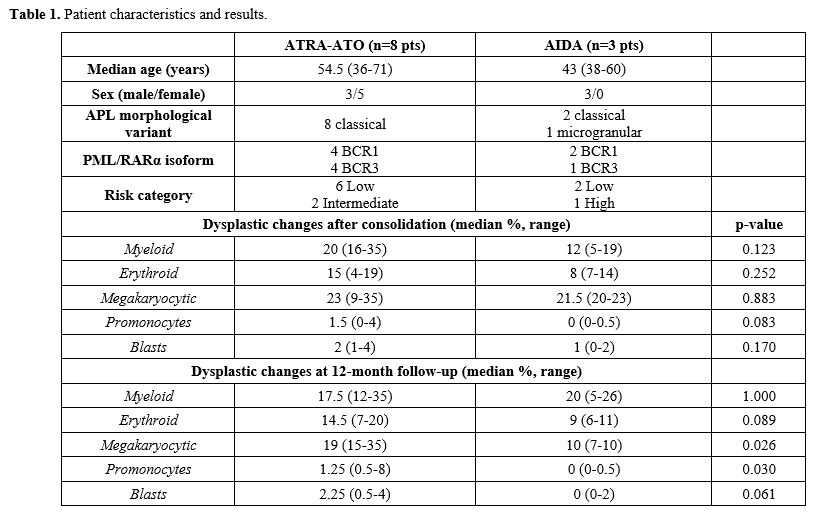
Ten patients (91%) presented a classical APL morphology, and only one (9%) had a microgranular type. PML::RARA isoforms were BCR1 in 6 patients (55%), whereas the remaining had the BCR3 isoform (45%). According to Sanz's risk score, 8 patients (73%) were diagnosed with low-risk, 2 (18%) intermediate-risk, and one (9%) with high-risk APL. Overall, 8 patients (73%) received ATRA/ATO, and 3 were treated with the AIDA protocol (27%). Patient characteristics and results are summarized in Table 1.
In the whole group, a moderate grade of dysplasia was observed at the consolidation time point in both the erythroid (15% vs. 8% in the ATRA/ATO and AIDA group, respectively) and granulocytic (20% and 12% in the ATRA/ATO and AIDA group, respectively) lineages. Meanwhile, a severe grade of dysplasia was observed for the megakaryocytic lineage (23% vs. 21,5% in the ATRA/ATO and AIDA groups, respectively).
Focusing the analysis on different treatment protocols adopted, at the end of consolidation, at least a moderate grade of dysplasia was observed in all hematopoietic lineages for both treatment protocols, except for the erythroid lineage treated with AIDA (in which dysplasia was mild). Furthermore, we observed a higher proportion of promonocytes [1,5% vs. 0% (p=0.083), respectively] and mastocytes [1% vs. 0% (p=0.084) in ATRA/ATO vs. AIDA treatment groups, respectively (Table 1).
At the 12-month follow-up time point, the grading of dysplasia was moderate for all three cell lineages for the ATRA-ATO group. At the same time, it was moderate for granulocytic lineage and mild for both erythroid and megakaryocytic lineages in the AIDA group. In particular, the rate of megakaryocytic dysplasia was significantly higher in ATRA-ATO vs AIDA treatment groups (19% vs 10%, respectively, p=0.026). Similarly, we confirmed an increased proportion of promonocytes in the ATRA-ATO vs AIDA treatment groups at the same time point (1.25% vs 0%, p=0.030). Furthermore, there was a trend for increased erythroid dysplasia (14.5% vs. 9%, p=0.089) and of the proportion of myeloblasts e (median 2.25% vs. 0%, p=0.061) in the ATRA-ATO vs. AIDA group, respectively. Of note is that all patients were alive at a median of 45 months of follow-up from diagnosis (range 33-112 months), in complete molecular remission, and with no alterations of blood cell counts, thereby configuring such cases as idiopathic dysplasia of unknown significance (IDUS), a condition characterized by the presence of dysplastic bone marrow features, without any significant cytopenia.[9]
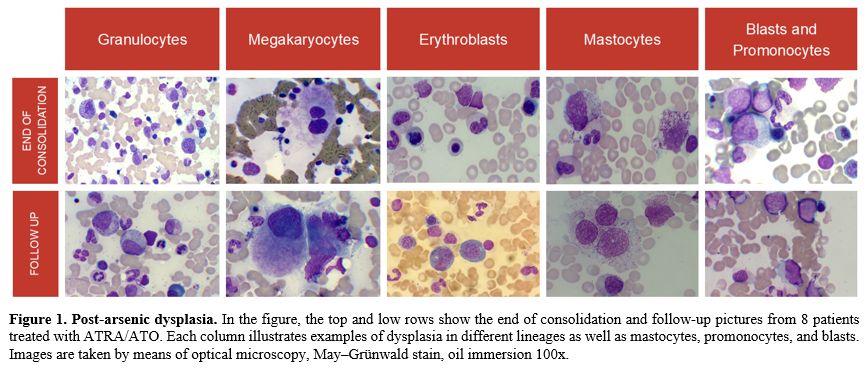
An important observation highlighted in our study is that while both ATRA-ATO and AIDA combinations induce treatment-related BM morphologic changes early in the treatment course (e.g., the end of consolidation), dysplastic features were mostly evident at long-term follow-up after ATRA-ATO exposure. Particularly, ATRA-ATO-treated patients presented moderate dysplasia of granulocytic and erythroid lineages and moderate/severe megakaryocytic dysplasia at both time points (Figure 1). These observations suggested that arsenic exposure may generate a characteristic IDUS form, which we will call “AIDUS” (ATO-induced dysplasia of uncertain significance).
As limitations of the study, we acknowledge the small sample size, the retrospective nature of the analysis, and the lack of cytogenetic and/or molecular data, which were not collected during patients' follow-up, lacking cytopenia, in the absence of suspicion of evolution into a secondary myeloid neoplasm.
However, our results may emphasize the need for careful morphological evaluation of long-term BM smears in patients exposed to ATRA/ATO, which may cause alarm and misdiagnoses of MDS in case of co-occurrence of not-related, poorly-investigated cytopenia. Indeed, long-term follow-up of the APL0406 trial has confirmed no deaths related to a secondary myeloid neoplasm in the ATO arm, compared to two cases (1 AML and 1 MDS) in the AIDA group.[3] Furthermore, to our knowledge, no cases of secondary myeloid neoplasms have been diagnosed after the exclusive ATRA-ATO combination, despite several events reported after chemotherapy-based treatment.[10-15] In this line, cytopenias during follow-up appear to be very rare in patients treated with ATRA-ATO.[16]
Notably, the last ELN guidelines, given the very low probability of relapse for non–high–risk patients, discourage prolonged BM minimal residual disease assessments in patients treated with ATRA-ATO.[17] IDUS after ATRA/ATO even begs the question of the actual need for long-term BM evaluations in patients with standard-risk APL treated with ATRA-ATO.
In conclusion, our findings of myelodysplastic changes in patients with APL treated with ATRA/ATO are intriguing but clinically non-significant to date. They do not indicate BM follow-up studies, which may turn out to be a confounding factor during patients’ follow-up. However, considering the inherent poisoning nature of arsenic on bone marrow and the lack of data on very long-term effects, prolonged follow-up of morphological peripheral blood smears could be considered.[18-20]
Acknowledgments
This work was supported by AIRC 5_1000 call “Metastatic disease: the key unmet need in oncology” to MYNERVA project, no. 21267 (Myeloid Neoplasms Research Venture AIRC. A detailed description of the MYNERVA project is available at http://www.progettoagimm.it). This work was also supported by the MUR-PNRR M4C2I1.3 PE6 project PE00000019 Heal Italia, PRIN grant P2022W25EA, Ministero della Salute, Rome, Italy, Bando Ricerca Finalizzata 2018, NET-2018-12365935: Personalized medicine program on myeloid neoplasms: characterization of the patient’s genome for clinical decision making and systematic collection of real-world data to improve quality of health care, to M.T.V.Data availability statement
The data supporting Table 1 are not publicly available to protect patient privacy. However, the corresponding author can provide access upon request.References
- F. Lo-Coco et al., 'Retinoic acid and arsenic
trioxide for acute promyelocytic leukemia.', N Engl J Med, vol. 369,
no. 2, pp. 111-21, Jul. 2013. https://doi.org/10.1056/NEJMoa1300874
- U.
Platzbecker et al., 'Improved Outcomes With Retinoic Acid and Arsenic
Trioxide Compared With Retinoic Acid and Chemotherapy in Non-High-Risk
Acute Promyelocytic Leukemia: Final Results of the Randomized
Italian-German APL0406 Trial.', J Clin Oncol, vol. 35, no. 6, pp.
605-612, Feb. 2017 https://doi.org/10.1200/JCO.2016.67.1982
- L.
Cicconi et al., 'Long-term results of all-trans retinoic acid and
arsenic trioxide in non-high-risk acute promyelocytic leukemia: update
of the APL0406 Italian-German randomized trial.', leukemia, vol. 34,
no. 3, pp. 914-918, Mar. 2020. https://doi.org/10.1038/s41375-019-0589-3
- M.
T. Voso et al., 'Acute promyelocytic leukemia: long-term outcomes from
the HARMONY project.', Blood, vol. 145, no. 2, pp. 234-243, Jan. 2025. https://doi.org/10.1182/blood.2024026186 PMid:39504485
- K.
P. Miller et al., 'Bone Marrow Findings in Patients With Acute
Promyelocytic Leukemia Treated With Arsenic Trioxide.', Am J Clin
Pathol, vol. 152, no. 5, pp. 675-685, Oct. 2019. https://doi.org/10.1093/ajcp/aqz087 PMid:31305869 PMCid:PMC6779253
- G.
Avvisati et al., 'AIDA (all-trans retinoic acid + idarubicin) in newly
diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie
Ematologiche Maligne dell'Adulto (GIMEMA) pilot study. Blood, vol. 88,
no. 4, pp. 1390-8, Aug. 1996. https://doi.org/10.1182/blood.V88.4.1390.bloodjournal8841390 PMid:8695858
- J.
W. Vardiman et al., 'The 2008 revision of the World Health Organization
(WHO) classification of myeloid neoplasms and acute leukemia: rationale
and important changes.', Blood, vol. 114, no. 5, pp. 937-51, Jul. 2009.
https://doi.org/10.1182/blood-2009-03-209262 PMid:19357394
- M.
G. Della Porta et al., 'Minimal morphological criteria for defining
bone marrow dysplasia: a basis for clinical implementation of WHO
classification of myelodysplastic syndromes.', leukemia, vol. 29, no.
1, pp. 66-75, Jan. 2015. https://doi.org/10.1038/leu.2014.161 PMid:24935723
- P.
Valent et al., 'Idiopathic bone marrow dysplasia of unknown
significance (IDUS): definition, pathogenesis, follow up, and
prognosis.', Am J Cancer Res, vol. 1, no. 4, pp. 531-41, 2011.
- R.
Latagliata et al., 'Therapy-related myelodysplastic syndrome-acute
myelogenous leukemia in patients treated for acute promyelocytic
leukemia: an emerging problem.', Blood, vol. 99, no. 3, pp. 822-4, Feb.
2002. https://doi.org/10.1182/blood.V99.3.822 PMid:11806982
- I.
Lobe et al., 'Myelodysplastic syndrome after acute promyelocytic
leukemia: the European APL group experience.', leukemia, vol. 17, no.
8, pp. 1600-4, Aug. 2003. https://doi.org/10.1038/sj.leu.2403034 PMid:12886249
- A.
Eghtedar et al., 'Incidence of secondary neoplasms in patients with
acute promyelocytic leukemia treated with all-trans retinoic acid plus
chemotherapy or with all-trans retinoic acid plus arsenic trioxide.',
Leuk Lymphoma, vol. 56, no. 5, pp. 1342-5, May 2015. https://doi.org/10.3109/10428194.2014.953143 PMid:25120050 PMCid:PMC4417657
- Z.
Wang et al., '[Therapy-related myeloid neoplasms after successful
treatment for acute promyelocytic leukemia: a report of four cases and
literature review].', Zhonghua Xue Ye Xue Za Zhi, vol. 40, no. 12, pp.
1008-1014, Dec. 2019.
- P.
Montesinos et al., 'Therapy-related myeloid neoplasms in patients with
acute promyelocytic leukemia treated with all-trans-retinoic Acid and
anthracycline-based chemotherapy.', J Clin Oncol, vol. 28, no. 24, pp.
3872-9, Aug. 2010. https://doi.org/10.1200/JCO.2010.29.2268 PMid:20625122
- D.
Gaut, J. Sasine, and G. Schiller, 'Secondary clonal hematologic
neoplasia following successful therapy for acute promyelocytic leukemia
(APL): A report of two cases and review of the literature.', Leuk Res
Rep, vol. 9, pp. 65-71, 2018. https://doi.org/10.1016/j.lrr.2018.04.005 PMid:29892552 PMCid:PMC5993360
- F.
Efficace et al., 'Long-term quality of life of patients with acute
promyelocytic leukemia treated with arsenic trioxide vs chemotherapy.',
Blood Adv, vol. 5, no. 21, pp. 4370-4379, Nov. 2021. https://doi.org/10.1182/bloodadvances.2021004649 PMid:34529768 PMCid:PMC8579253
- M.
A. Sanz et al., 'Management of acute promyelocytic leukemia: updated
recommendations from an expert panel of the European LeukemiaNet.',
Blood, vol. 133, no. 15, pp. 1630-1643, Apr. 2019. https://doi.org/10.1182/blood-2019-01-894980 PMid:30803991 PMCid:PMC6509567
- D.
Westhoff, R. Samaha, and A. J. Barnes, 'Arsenic intoxication as a cause
of megaloblastic anemia', Blood, vol. 45, no. 2, pp. 241-246, Feb.
1975. https://doi.org/10.1182/blood.V45.2.241.241 PMid:1120185
- H.
Pralle and F. Manz, 'Influence of chronic arsenic poisoning on bone
marrow morphology', Blut, vol. 50, no. 1, pp. 51-54, Jan. 1985. https://doi.org/10.1007/BF00319771 PMid:3967100
- J.
Feussner, J. Shelburne, S. Bredehoeft, and H. Cohen, 'Arsenic-induced
bone marrow toxicity: ultrastructural and electron- probe analysis',
Blood, vol. 53, no. 5, pp. 820-827, May 1979. https://doi.org/10.1182/blood.V53.5.820.bloodjournal535820 PMid:435641