Nevertheless, the relapsing nature of the disease imposes continuous treatment exposure, and virtually all patients develop subsequent refractoriness to previously effective drugs.[5] Noteworthy, the prognostic scenario of patients who are refractory to an anti-CD38 antibody, an immunomodulatory agent (IMiD), and a proteasome inhibitor (PI) (namely triple-class refractory MM) is particularly poor, with estimated OS rates of less than one year.[6-8]
In this setting, immune-based therapies aimed at redirecting patients’ T-cells against specific tumor antigens proved to be effective, leading to the introduction of chimeric antigen receptor (CAR) T-cells and bispecific antibodies (BsAbs) into clinical practice.[9-15] Although conceptually similar, these two strategies differ in a range of features, so that accurate selection of the right patient for the appropriate treatment at the proper time is mandatory.[16] Moreover, the overlapping regulatory indications of CAR T-cells and BsAbs, coupled with the inherent development of resistance by different biological mechanisms, pose the issue of sequencing more than one immune-based therapy after another, with the intent of maximizing efficacy.[17] In this article, we will briefly review the main results from the most relevant registrational trials, as well as promising combination strategies and new agents under advanced investigation. Henceforth, we will dedicate a special focus to the current body of knowledge regarding patient selection and treatment sequencing with these cutting-edge therapies.
CAR T-Cell Therapy in Multiple Myeloma
CAR T-cells are autologous T-cells transduced with a lentiviral or retroviral vector that carries a gene encoding for a CAR. This latter consists of an extracellular targeting domain without Major Histocompatibility Complex (MHC) restriction, usually derived from a single-chain variable fragment (scFv) of a monoclonal antibody; the extracellular domain is linked to an intracellular signaling domain that includes a CD3ζ activation domain and a co-stimulatory domain (mainly 4-1BB). After transduction, these cells are expanded and then re-infused into patients, upon lymphodepleting chemotherapy. Once infused, CAR T-cells engage with the tumoral antigens independently of human leukocyte antigen (HLA), release cytokines, lyse target cells, and proliferate in vivo through the action of the co-stimulatory domain.[18,19] Thus far, the main targets explored with available constructs include the B-cell maturation antigen (BCMA) and the G protein–coupled receptor class C group 5 member D (GPRC5D). Other products designed to recognize further antigens that can be targeted either alone or in combination with B-cell maturation antigen (BCMA) are under development. The efficacy results of the most relevant CAR T-cells trials are summarized in Table 1.Idecabtagene vicleucel. Idecabtagene vicleucel (ide-cel) is a BCMA-directed, second-generation CAR T-cell construct.
Ide-cel was first explored in patients exposed to at least 3 previous lines of therapy in the phase II KarMMa trial (NCT03361748). Overall response rate (ORR) was 73%; 52% of patients obtained a very good partial response (VGPR), or better. Median progression-free survival (mPFS) was 8.8 months (mos) and mOS was 19.4 mos.[9]
Subsequently, the phase III KarMMa-3 trial (NCT03651128) enrolled patients exposed to two to four previous lines of therapy, who were randomized to receive either ide-cel or standard of care (SOC) therapy (including daratumumab plus either bortezomib-dexamethasone, or pomalidomide-dexamethasone [DPd], elotuzumab-pomalidomide-dexamethasone [elo-Pd], ixazomib-lenalidomide-dexamethasone, or carfilzomib-dexamethasone [Kd]). ORR was 71% for ide-cel vs 42% for SOC; CR rate was 44% vs 6%, respectively. Ide-cel demonstrated a PFS benefit with a mPFS of 13.8 mos vs 4.4 mos. Instead, no OS benefit was shown, with a mOS of 41.4 mos and 37.9 mos, respectively. In this sense, however, it should be underscored that cross-over to experimental therapy was permitted in this trial. Indeed, 56% of patients received Ide-cel as subsequent therapy, after progressing with SOC. When adjusting for crossover, the ide-cel arm portended a trend toward superior OS when compared to the SOC arm (mOS 41.4 mos vs 23.4 mos, respectively).[11,20]
Based on these results, ide-cel is approved by the European Medicines Agency (EMA) and the Food and Drugs Administration (FDA) for the treatment of adult patients with relapsed and refractory multiple myeloma (RRMM) who have received at least two prior therapies, including an IMiD, a PI, and an anti-CD38 antibody, and who have demonstrated disease progression on the last therapy.
Ciltacabtagene autoleucel. Ciltacabtagene autoleucel (cilta-cel) is a CAR-T agent expressing two BCMA-targeting single-domain antibodies designed to confer high avidity.[10]
CARTITUDE-1 (NCT03548207) is a phase Ib/II study that explored cilta-cel for patients previously exposed to at least 3 lines of therapy or double refractory to a PI and an IMiD. ORR was 97.9%, with a stringent complete response (sCR) rate of 82.5%. At 27 mos, PFS was 54.9% and OS 70.4%. At a subsequent update, mPFS for the cilta-cel arm was 34.9 mos.[10,21,22]
Considering the results of the CARTITUDE-1 trial, the phase III CARTITUDE-4 trial (NCT04181827) was designed to compare cilta-cel vs SOC therapies (i.e., pomalidomide-bortezomib-dexamethasone [PVd] or DPd, at physician’s discretion) in patients exposed to 1-3 prior lines of treatment and who were refractory to lenalidomide. Efficacy results at 3 years follow-up showed an ORR of 84.6% in the cilta-cel arm vs 67.3% in the SOC arm, with ≥VGPR being 81.2% vs 45.5% and ≥CR 76.9% vs 24.2%, respectively. PFS was significantly better in the cilta-cel arm (mPFS not reached [NR] vs 11.8 mos; PFS at 30 mos 59.4% vs 25.7%). An OS advantage at 30 mos was also showed (76.4% vs 63.8%, respectively). Consistently, minimal residual disease (MRD) negativity rates with a minimal sensitivity of 10-5 were superior in the cilta-cel arm (62% vs 18.5%, respectively), in an intention-to-treat analysis.[12,23]
Currently, cilta-cel is approved by FDA and EMA for the treatment of adult RRMM patients who have received at least one prior therapy, including IMiD and a PI, have demonstrated disease progression on the last therapy, and are refractory to lenalidomide.
A controlled and randomized head-to-head comparison between ide-cel and cilta-cel is still lacking. However, a retrospective, multicenter analysis of 586 pts treated with either cilta-cel or ide-cel was recently conducted. The two cohorts were well-balanced and represented different baseline characteristics evenly. Cilta-cel was proven to confer better efficacy in terms of response rates, PFS, and OS in most of the patient subpopulations, though associated with a more burdensome toxicity profile in terms of high-grade CRS, delayed neurotoxicity, and infections.[24]
Anitocabtagene autoleucel. Anitocabtagene autoleucel (anito-cel, previously known as CART-ddBCMA) is an anti-BCMA CAR T-cell construct modeled to express a D-domain binder between the CD8 hinge-region and the transmembrane domain. The D-domain is of synthetic and non-antibody origin, facilitating the transduction of activation signals, while reducing tonic signaling. In the iMMagine-1 phase I/II trial (NCT05396885), patients exposed to at least three previous line of therapy and to at least a PI, an IMiD, and an anti-CD38 antibody, received anito-cel, with a dose-finding approach. Collectively, ORR was 100%, CR rate 76%, while 24-mos-PFS was 56%. MRD negativity with a minimum sensitivity of 10-5 was reached in 89% of all evaluable patients.[25] Furthermore, a phase III, randomized, registrational trial (iMMagine-3, NCT06413498) is ongoing to confront anito-cel vs SOC therapies (i.e., PVd, DPd, Kd, or daratumumab plus Kd) in patients with RRMM previously exposed to 1-3 lines of therapy and at least to an anti-CD38 and an IMiD.[26]
Anti GPRC5D CAR T-cells therapy. MCARH109 is a CAR T-cell construct with a GPRC5D single-chain variable fragment. MCARH109 safety and efficacy were explored in a phase I trial (NCT04555551) that enrolled triple-class exposed patients with RRMM with 3 or more previous lines of treatment. Interestingly, 59% of patients were exposed to a prior anti-BCMA therapy and 47% to a prior CAR T-cell therapy. ORR was 71% in the whole population, 70% in BCMA-exposed patients, and 75% in BCMA CAR T-cells treated patients.[27,28]
Arlocabtagene autoleucel (arlo-cel) (CC-95266, formerly BMS-986393) is another GPRC5D-targeting autologous CAR T-cell agent. Preliminary data from a phase I trial (NCT04674813) enrolling triple-class exposed patients with three or more previous lines of treatment (49% of whom had received a prior BCMA-targeted therapy, including a BCMA-directed CAR T-cells therapy in 38% of them) showed efficacy and a favorable safety profile. At almost 15 mos median follow-up, ORR was 87% (38% CR) in the whole population and 79% in patients previously treated with anti BCMA therapy, while mPFS was 14.5 mos. Also, long-term safety data revealed a manageable profile, supporting BMS-986393 as a potential treatment for RRMM and further research (e.g., the ongoing phase II QUINTESSENTIAL study, NCT06297226).[29,30] Interestingly, preliminary data from patients with 1-3 prior lines of therapy including a PI and an IMiD are also encouraging, with high rates of response that deepened over time and no new safety concerns, though the follow-up is still limited.[31] Moreover, a phase III, randomized, registrational clinical trial (QUINTESSENTIAL-2, NCT06615479) is ongoing to confront arlo-cel vs SOC therapies in patients with lenalidomide-refractory RRMM exposed to 1-3 lines of therapies including an anti-CD38, a Pi and an IMiD.
Bispecific Antibodies in Multiple Myeloma
BsAbs are monoclonal antibodies designed with two fragment antigen-binding (Fab) arms capable of creating an immune synapsis between T-cell receptor (CD3) and a tumor cell antigen, thus leading to T-cells activation without MHC restriction. As of today, almost all BsAbs contain a fragment-crystallizable (Fc) domain that adds stability, increases the half-life of the molecule and induces T-cell- and complement-dependent cytotoxicity.[32] At present, BsAbs targeting BCMA, GPRC5D and the Fc receptor-like 5 (FcRL5) have demonstrated remarkable clinical activity in triple-class refractory patients, granting approval to some of these products, while several newer agents are under study. The efficacy results of the most relevant BsAbs trials are summarized in Table 2.Teclistamab. Teclistamab (Tec) is a subcutaneous BsAb that simultaneously targets CD3 on T-cells and BCMA on myeloma cells.
Tec safety and efficacy were evaluated in the phase I/II trial MajesTEC-1 (NCT04557098) that enrolled triple-exposed RRMM patients. The ORR was 63%; CR or better rates were 46.1%. mPFS was 11.4 mos and mOS was 22.2 mos. At a median follow-up of 30.4 mos,PFS for patients in CR was estimated to be 61%.[13,33]
Tec is being investigated alone or in combination with daratumumab in a phase III randomized clinical trial(MajesTEC-3, NCT05083169) against SOC therapies (i.e., DPd or DVd, as per investigator’s choice) in patients with RRMM exposed to 1-3 lines of therapy including an IMiD and a PI.[34]
At present, Tec is approved by EMA as monotherapy in patients with RRMM who have received at least three prior therapies, including an IMiD, a PI, and an anti-CD38 antibody, and have demonstrated disease progression on the last therapy. FDA approval includes patients previously exposed to four prior lines of therapies.
Elranatamab. Elranatamab (Elra) is a subcutaneous humanized BsAb that targets both BCMA and CD3.
Elra has been studied in the MagnetisMM-1 phase I trial (NCT03269136) and subsequently in the phase II MagnetisMM-3 trial (NCT04649359) that enrolled triple-class exposed RRMM patients. After a median follow-up of about 28 mos, the ORR was 61.0%, with 37.4% CR or better. Median PFS and OS were 17.2 mos and 24.6 mos, respectively. The probability of maintaining a response at 24 mos was estimated to be 87.9% for patients in CR or better.[14,35]
At present, Elra is being investigated alone or in combination with daratumumab in a phase III randomized clinical trial (MagnetisMM-5, NCT05020236) against SOC (i.e, DPd) in RRMM patients who have received at least 1 prior line of therapy including lenalidomide and a PI.[36]
Elra is currently approved by FDA and EMA as monotherapy for the treatment of adult patients with RRMM, who have received at least three prior therapies, including an IMiD, a PI, and an anti-CD38 antibody and have demonstrated disease progression on the last therapy.
Linvoseltamab. Linvoseltamab (REGN5458) is an intravenous fully human BCMAxCD3 BsAb designed to have minimal immunogenicity together with favorable molecular stability and pharmacokinetic properties.[37]
The phase I-II LINKER-MM1 (NCT03761108) trial showed encouraging results, with 70.9% ORR, 63.2% ≥VGPR, and 49.6% ≥CR in patients who received the full dose of 200 mg. 12-mos PFS and OS were 70.0% and 75.3% respectively.[37] Currently, an open-label, randomized phase III trial, LINKER-MM3 (NCT05730036), is evaluating safety and efficacy of linvoseltamab monotherapy compared with SOC (i.e, elo-Pd) in RRMM patients.
To date, linvoseltamab is not approved by regulatory agencies.
Talquetamab. Talquetamab (Tal) is a subcutaneous bispecific immunoglobulin G4 (IgG4) antibody that targets GPRC5D with a scaffold designed to minimize Fc-receptor binding to both GPRC5D and CD3.
Tal has been evaluated in the phase I/II trial MonumenTAL-1 study (NCT03399799/NCT04634552) enrolling triple-exposed patients with ≥3 previous lines of therapy.[15] In the 0.8 mg/kg every other week (Q2W) cohort, ORR was 69.5%; ≥VGPR and ≥CR rates were 59.1% and 40.3% respectively. mPFS was 11.2 mos.[38,39]
Moreover, Tal is being investigated in combination with daratumumab with or without pomalidomide in a phase III, randomized, clinical trial (MonumenTAL-3, NCT05455320) against SOC (i.e, DPd) in patients with RRMM exposed to at least 1 prior line and at least to an IMiD and a PI.[40]
Tal is approved by EMA as monotherapy at the dose of 0.4 mg/kg weekly or 0.8 mg/kg Q2W for the treatment of patients with RRMM, who have received at least 3 prior lines of therapy, including an IMiD, a PI, and an anti-CD38 antibody and have demonstrated disease progression on the last therapy. FDA approved Tal for the treatment of patients with RRMM, who have received at least 4 prior lines of therapy.
Cevostamab. Cevostamab is intravenous an IgG1-based T-cell-engaging BsAb that targets FcRH5 on myeloma cells and CD3 on T cells.[41]
The ongoing open-label, multicenter phase I/II trial CAMMA-2 (CO43476; NCT05535244) is evaluating cevostamab in triple-class refractory RRMM patients who had previously received anti-BCMA agents for whom no established therapy was available, appropriate, or tolerable. Interestingly, 57% of patients had received ≥1 prior BCMA targeted therapy and 24% of them had received ≥1 prior BsAb. ORR was 43.1%, VGPR o better was 25.7%. ORR was 30.2% in patients with ≥1 prior BCMA-targeted therapy and 60.6% in those without. ORR was 30.0% in patients with ≥1 prior BsAb, 33.3% with ≥1 prior CAR T-cell, and 41.2% with ≥1 prior antibody-drug conjugate (ADC).[41]
To date, cevostamab is not approved by regulatory agencies.
BsAbs and CAR T-Cells: Toxicity Profile
CAR T-cells and BsAbs share a similar toxicity profile, arising from the production of inflammatory cytokines. Thus far, the most relevant safety alerts are represented by cytokine realizing syndrome (CRS) and immune effector cells associated neurotoxicity syndrome (ICANS), though other common and equally distinctive toxicities associated to these promising immunotherapies are emerging, including cytopenias and infections.CRS is a systemic inflammatory reaction presenting with fever and possibly with hypotension and hypoxemia that might require intensive life support.[42] After few days from CAR-T cell infusion, CRS is common (84-95%), with some G3/4 events (4-5%).[9,10] BsAbs are associated with a slightly lower CRS rate (75-79%), while G3 events are exceedingly rare. The onset occurs mainly during the step-up dosing and at the first full dose.[13-15,37,41]
ICANS is a neurotoxic syndrome with a poorly understood pathophysiology in which disruption of the brain blood barrier and various pro-inflammatory cytokines might have a role.[43] ICANS typically manifests as a toxic encephalopathy presenting with word-finding difficulty, confusion, dysphasia, aphasia, impaired fine motor skills, and somnolence. More severe cases are characterized by seizures, motor weakness, cerebral oedema, and coma. In the anti-BCMA CAR T-cells setting, the incidence of neurotoxic events is 18-21% with some G3/4 events (3-9%).[9-12] Furthermore, cilta-cel portended a characteristic Parkinson-like syndrome, affecting 6% of patients in CARTITUDE-1 and 1 patient in CARTITUDE-4, together with cranial nerve palsy in 9% of patients in the latter study.[10,12] As anti-GPRC5D CAR T-cells are under development, one safety signal in terms of neurotoxicity is the association with some cases of cerebellar ataxia.[28,30] Meanwhile, BsAbs are associated with very low rate of ICANS (3-13%), all being grade 1 or 2. Of note, in the MagnetisMM-3 trial, 17% and 13% of patients developed motor dysfunction or sensory neuropathy, respectively.[13-15,37,41]
Beyond these well-known side effects, infections are common in patients affected by MM, especially under treatment.[44] In the context of CAR T-cells trials, the rate of infection ranged between 58% and 69%, G3/4 being 20-22%. However, it is important to highlight that in the KarMMa-3 and CARTITUDE-4 trials, the incidence of infections was similar between the experimental and the SOC arms.[9-12,20,21] Similarly, anti-BCMA BsAbs are also burdened by a high incidence of infections (74-79%), G3/4 ranging from 35% to 55%, with some mortality signals.[33,45] Notably, this risk can be effectively mitigated with intravenous immunoglobulin (IVIG) supplementation and schedule modifications from weekly (QW) to Q2W.[33,46] Of note, the rate of infections is lower with non-BCMA BsAbs. Indeed, high-grade events are much rarer, and no grade 5 infections are reported: overall, infections range between 60-70%, with G3/4 around 20-22%.[38,39,47]
Cytopenias are frequent and their incidence is higher in patients treated with CAR T-cells, as compared to BsAbs. In particular, G3/4 neutropenia is reported in 90% of patients treated with CAR T-cells, in 65% of patients treated with anti-BCMA BsAbs, and in 30% of patients treated with non-anti-BCMA BsAbs, whereas G3/4 thrombocytopenia affected about 55% of patients treated with CAR T-cells and 20% of patients treated with BsAbs.[9,21,29,31,33,37,38,41,45] Noticeably, cytopenias during BsAbs therapy is often quickly reversible with dose delays and growth factor support. Instead, prolonged cytopenias after CAR T-cell therapy were reported, configuring a specific entity denominated immune effector cell–associated hematotoxicity (ICAHT).[48] ICAHT is characterized by cytopenia persisting long after the resolution of clinical CRS. It correlates with tumor and inflammatory burden, as deciphered by the dedicated risk-score CAR-HEMATOTOX, and its occurrence portends decreased survival.[49,50]
In addition, secondary primary malignancies (SPMs) are reported after CAR T-cells therapies and the FDA has published a warning about the risk of development of T-cell lymphomas.[51] In MM CAR T-cells trials these data do not appear to be confirmed with the current follow up. In the KarMMa-3 study, the incidence per 100 patient-years of SPMs was comparable between the ide-cel and SOC (3.6 vs 4.1 respectively). No SPMs of T-cell origin was reported in the ide-cel arm. In the CARTITUDE-4 trial, the incidence of SPM was 4.3% in cilta-cel arm and 6.7% in SOC, while one peripheral T-cell lymphoma was reported.[12,20]
Furthermore, owing to target expression on keratinized tissues, anti-GPRC5D TCR are affected by on target/off tumor toxicities in particular skin related (73%), nail related (53%), dysgeusia (71%) and weight loss (≥10% from baseline) (34%). Additionally, the anti-GPRC5D CAR-T cells trials reported few cases of cerebellar ataxia (MCARH109: 2/17 patients; arlo-cel: 2/70 patients).[27-29,39]
In response to the emergence of these adverse events, the International Myeloma Working Group (IMWG) has issued specific recommendations on the prevention and management of these TCR toxicities.[52,53]
The toxicity profile of the most relevant TCR agents is summarized in Table 3.
Challenges of the Immunotherapy Era: Patient Selection and the Need for Sequential Treatments
The efficacy leveraged by TCR agents, as described in the previous sections, marked a step forward in the previously difficult-to-treat triple-class-refractory MM scenario.[54] Importantly, such efficacy is foreseen to be fostered by moving these therapies in earlier line settings, or by combination strategies.[55,56] However, several aspects need to be weighted in order to maximize their action, since a definitive curative intent is mostly far from being met for the majority of the patients.First of all, it is conceivable that the first TCR agent a patient receives will be the one benefiting the most; thus, it needs to be carefully selected. In this sense, clinical (both patient- and tumor-wise) and logistical aspects ought to be considered. Moreover, treatment selection should account for the potential need of sequential exposure to different TCR therapies, whose efficacy may be mutually influenced. Indeed, the critical themes of interest in sequencing TCR treatments are the features of prior TCR exposure, the mechanisms of resistance causing the failure of previous agents possibly implied in the efficacy of subsequent lines), (evidence from clinical trials, and real-life observations regarding the effectiveness of specific sequencing models. Thus, patient selections and sequencing of TCRs are the subject of intensive research and clinical review. Recently, in-depth recommendations from the IMWG were published.[57]
Patient selection: practical and clinical aspects of interest. The choice of the first TCR agent a patient is receiving should integrate both patient- and tumor-specific aspects, alongside with logistical implications.[16,17,57] Importantly, the first limitation of CAR T-cells therapy is the time imposed by the manufacturing process (i.e., the time running between leukapheresis and infusion of the product, conceptually addressed as vein-to-vein period). Additionally, a brain-to-vein period is also implied, i.e., the time between the physician’s referral and access to an accredited tertiary center capable of ensuring CAR T-cells administration.[57] As brain-to-vein and vein-to-vein periods are highly variable in their duration, with the present technology of CAR T-cells manufacturing and spread of CAR T-cells centers in western countries, they are estimable in 1-2 months.[16,58-60] In this sense, tumor progression pace should be indolent (or efficiently controlled) enough to bridge the patient through the process, without running into clinical deterioration or development of uncontrollable progression.[16,17,57] In fact, efficacy of CAR T-cells was demonstrated to be potentially hampered by the extent of tumor burden, which ultimately also affects morbidity in terms of CRS and ICANS.[21,61-64] Brain-to-vein durations and referral limitations could be lessened by more efficient manufacturing technologies, increased number of facilities accredited for CAR T-cells administration in the national territory, and manufacturing of academic-driven products.[65-67] In this regard, it must be remembered that up to 10-20% of referred patients could not receive the CAR T-cell product after apheresis due to uncontrolled disease progression, or manufacturing failure.[58-60]
Expectedly, the types of therapy a patient receives through the CAR T-cells process are of great importance. Specifically, the therapy potentially needed after first referral is addressed to as holding therapy, whose purpose should be to impede further tumor progression without interfering with the manufacturing process and CAR T-cells anti-tumor capacity.[16,17,57] Possibly, it should be based on agents to which the patient is not refractory or recently exposed, and drugs negatively interfering with lymphocytes’ biology, such as alkylators, should be avoided.[68] In this setting, an interesting scenario is represented by the possibility of fostering lymphocytes’ fitness in terms of those qualities demonstrated to positively impact the efficacy of CAR-T constructs, such as ratios of naïve and memory T-cells.[69,70] Interestingly, is has been proposed that IMiDs are capable to increase T-cell fitness in terms of proliferation and persistence potential.[71,72] With the same rationale, CELMoDs (Cereblon E3 Ligase Modulators) are being investigated with the purpose of improving the quality of T-cell response before and after TCRs.[73,74] Then, therapy is to be held two to four weeks before leukapheresis itself, as suggested by the IMWG committee, even though robust evidence is lacking.[57]
On the other hand, the therapy potentially needed after leukapheresis and before product infusion (i.e., during vein-to-vein time) is referred to as bridging therapy.[57] It should impede further tumor progression and possibly reduce its burden. Under these terms, cytotoxic therapy as debulking solution could be used, although it has been demonstrated to correlate with inferior outcomes.[75] In addition, it has been shown that overall response to bridging therapy correlates with treatment outcome. However, bridging therapy itself portends shorter survival, especially when ineffective, possibly as a surrogate of a more aggressive disease.[76,77] Also, quite differently from the holding therapy, it is captivating that bridging strategies based on BsAbs are soon to be further investigated, with the rationale that they might be implicated in the amelioration of T-cell repertoire in terms of memory and persistence. In this regard, growing interest in the use of talquetamab is emerging.[78,79] The IMWG committee recommended a two-week washout of bridging therapy before CAR T-cells infusion.[57]
By contrast, BsAbs inherently represents an off-the-shelf strategy that is not hampered by manufacturing time and is less limited by reduced accessibility. Thus, when dealing with tumor progressions that require immediate intervention, it seems rational to prefer BsAbs. Nevertheless, it should be stressed that even BsAbs demonstrated less efficacy in high tumor burden diseases, variably represented by extramedullary disease, elevated soluble BCMA and bone marrow plasma-cells percentage.[13,14]
Another key aspect to be considered in patient selection concerns the specific toxicity profiles of TCRs. Frail patients are usually not deemed eligible for CAR T-cells, upon the assumption of insufficient organ function to cope with the burdensome impact of CRS, ICANS, and prolonged cytopenia.[16,17] This adds to the real-life awareness of an increased incidence of high-grade events in the frail population.[80,81] Even though robust standardization is lacking, patients are usually screened for eligibility to CAR-T therapies based on pre-immunotherapy frailty scores. Interestingly, it must be noted that real-life experiences with CAR-T administrations showed equal efficacy and safety for patients not meeting inclusion criteria in registrational trials, underpinning their feasibility in less selected patients.[58-60] On the other hand, the lower incidence of severe CRS, ICANS and prolonged cytopenia portended by BsAbs makes them a potentially better option for frail patients,[17,57] though the inherent infectious risk warrants prudence when referring a patient with a history of recurrent and/or severe infections.[82]
CAR T-cells and BsAbs also differ in terms of logistical burden on health-care facilities, caregivers, and patients themselves.[16,17,50] While CAR T-cells require a complex organization in terms of accessibility, administration, and early management of toxicities, it is indeed a one-shot therapy. As a result, patients could undergo prolonged periods of remission, with reduced access to the hospital and ancillary therapies. In this sense, improved quality of life leading to significant and meaningful improvements in relevant symptoms, fatigue, physical functioning, and overall health status has been demonstrated in both ide-cel and cilta-cel trials, underscoring the favorable impact of such a strategy on patient’s well-being.[83,84] Additionally, such a treatment-free interval could possibly leverage the recovery of T-cells, which could be eventually beneficial in a potential subsequent TCR therapy at relapse.[85,86] At the same time, BsAbs do not require access to a tertiary center, although the schedule of administration until-remission, alongside the need for supportive therapies to prevent infectious complications, requires frequent and facilitated access to the hospitals. Thus, though BsAbs were also proved to be beneficial in terms of improved patients’ quality of life in registrational trials, this may be impacted by the burden of continuous therapy.[87-89]
Sequencing of T-cell redirecting therapies. CAR T-cells and BsAbs have marked a paradigm shift in the treatment of RRMM. However, they still lack curative potential in most cases, especially in the late line setting they were approved for in the first instance.[90-92]
Intense threads of clinical research are also ongoing to boost their beneficial effects, in terms of earlier line positioning and combinatorial strategies.[12,20,34,36,40,93-96] As such, when referring a patient to a TCR agent, the treating physician should foresee the chance of prescribing more than one strategy after another. This raises the issue of finding an optimal sequencing model, ultimately intended to enhance the activity of a single agent by virtue of previous exposure to TCRs. Therefore, the adoption of an ideal sequencing requires an adequate understanding of the mechanisms of resistance.[86,97]
In this regard, CAR T-cells and BsAbs share some mechanisms of resistance while differing in others. For instance, they are both impacted in their functioning by detrimental tumor specific aspects, such as high-risk cytogenetics, elevated ferritin, extramedullary disease, plasma cells leukemia, or elevated soluble BCMA.[13-15,21,98,99] They are also accustomed by microenvironment-driven resistance in terms of immunosuppressive effect of myeloid derive stromal cells (MDSC), increased regulatory T cells (T-regs) and inhibitory cytokine’s pattern.[100-102] Furthermore, an important mechanism of resistance is represented by persisting or acquired exhaustion of T-cell functioning, especially in regard of loss of naïve and memory repertoire.[103-105] In these terms, BsAbs and CAR T-cells do differ, given the persisting T-cell activation fostered by the first as compared to the time-limited anti-tumor effect of the second, raising the chance for a fixed duration schedule of BsAbs, with the intent of limiting immune system exploitation.[106,107] The different treatment pressure also makes sense for the diverse emerging of resisting clones. In fact, while antigen loss represents a rare event after CART therapies, in relation to their time-limited effect, it is described to account for about 50% of relapses after exposure to BsAbs.[86,108] Moreover, agents targeting different antigens may develop specific pattern of resistance, being complete antigen downregulation specific of GPRC5D, while BCMA more often undergoes epitopes mutation, in light of their respective different role in plasma-cells biology.[27,86,108,109] It is also believed that selective and persisting treatment pressure gives rise to resistant clones by favoring the emerge of acquired mechanism of resistance, rather than the selection of pre-existing resistant sub-clones, underpinning the importance of surveillance of clone populations during treatment.[110]
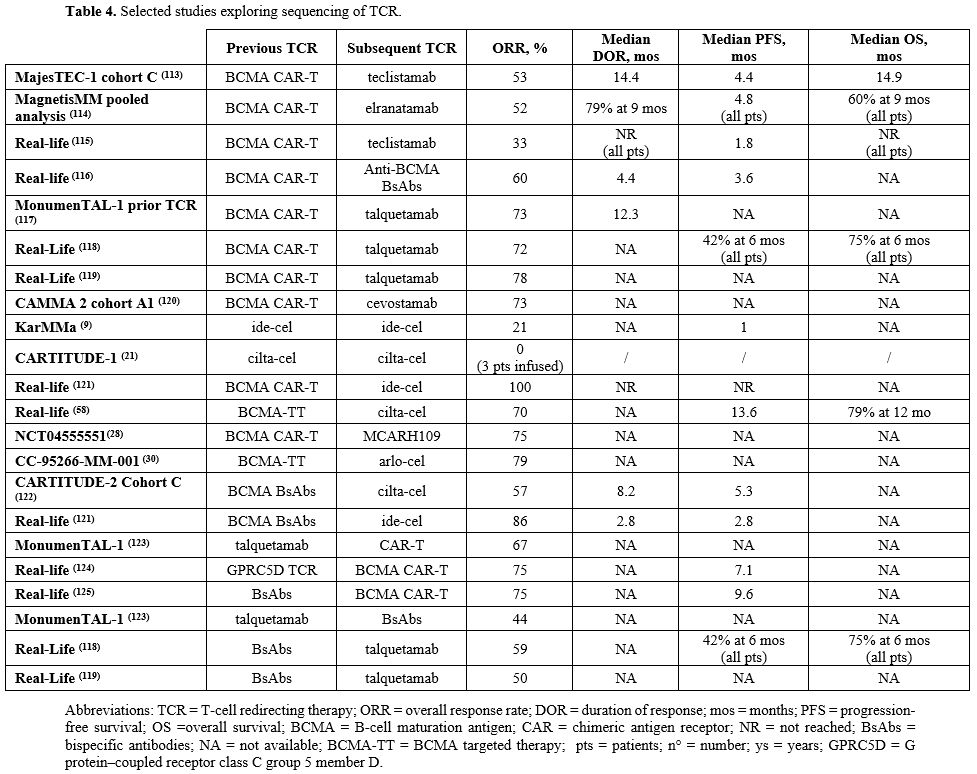
In addition to an in-depth comprehension of the mechanisms of resistance, the collection of robust clinical data with the final intent of deciphering the efficacy and feasibility of specific sequencing models is of crucial importance. Conceptually, real-life analyses have shown that sequencing TCRs is feasible and outperforms other treatment modalities, with the chance of prolonged disease control in difficult-to-treat patients. In a retrospective US cohort of 79 CAR T-cells-treated patients, 35 received a TCR agent at any given timepoint after CAR T-cells. The ORR was 91.4%, mPFS of salvaged patients was 9.1 mos and OS was not reached at 21 mos of follow-up.[111] Similarly, a retrospective cohort of 58 BsAbs-treated patients (49 with anti-GPRC5D, 9 with anti-BCMA agents) showed promising outcomes with subsequent TCR exposure (19 patients: 10 BsAbs, 9 CAR T-cells). ORR, PFS and OS were 84%, 28.9 mos and not reached, respectively.[112] Both studies showed consistently worse outcomes in conventionally treated cohorts. The efficacy results of the most relevant sequencing models are summarized in Table 4.
1-BsAbs after CAR T-cells therapies. CAR T-cell therapy was developed for patients affected by RRMM before the availability of BsAbs. As such, a sufficient amount of data is available from registrational trials and real-life observations regarding the CAR T-cells followed by a BsAb sequencing model.
Tec was studied in patients previously exposed to BCMA-targeted treatment in the cohort C of MajesTEC-1 trial. The ORR was 52.5%, the mPFS was 4.5 mos and the mOS 15.5 mos. Efficacy was similar in patients with prior BCMA-targeted ADC and CAR T-cells.[113] Also, studies in the MagnetisMM program (MM-1, MM-3, MM-9) enrolled patients treated with prior BCMA-directed therapies. In CAR T-cells pre-treated patients, the ORR was 52%, and mPFS in the whole population was 4.8 mos.[114]
Data arising from real-life experience of patients treated with Tec or Elra confirmed the evidence derived from clinical trials. Nevertheless, while ORR seems independent from prior anti-BCMA CAR T-cell exposure, the PFS is consistently lower in patients previously treated with an anti-BCMA CAR-T therapy. All in all, it is not clear whether the time elapsing between CAR-T cell infusion and the subsequent therapy with an anti-BCMA BsAb may affect ORR and PFS.[115,116]
MonumelTAL-1 trial showed good efficacy of Tal in patients previously exposed to anti BCMA CAR-T, validating the principle of changing the target. Particularly, in the subgroup of patients previously exposed to CART cell therapy, an ORR of 72% and a median duration of response (mDoR) of 12.3 mos were noticed. Furthermore, the ORR was comparable in patients who received CAR-T as immediate prior line vs any prior line of therapy before Tal (75.9% vs 71.4%, respectively).[117] The few real life experiences available when Tal was used after an-anti BCMA CAR T-cell therapy confirmed the good rate of response (with ORR of 72-78%) obtained in the MonumelTAL-1 trial.[118,119]
The CAMMA 2 study included the cohort A1 where cevostamab was given to patients exposed to a prior anti-BCMA targeting ADC or CAR T, showing an encouraging ORR of 73% in the group with prior CAR T.[120]
Overall, data arising for selected cohorts of pivotal trials and from real life experiences show good efficacy of BsAbs in patients relapsed after an anti-BCMA CAR T-cell therapy, especially using a BsAb with a different target. Biological insights could possibly rely on the prolonged off-therapy period, that poses the chance for immunological reconstitution of T-cells populations, eventually hesitating in a better performance of BsAbs-based therapies.[85,86,116]
2-Sequential CAR T-cells therapies. Data regarding the sequential use of more than a CAR T-cell product after another are limited. In this sense, the re-infusion of both ide-cel and cilta-cel at subsequent relapse was studied in restricted cohorts of the KarMMa-1 and CARTITUDE-1 trials, however with unsatisfactory results.[9,21] Efficacy of a different CAR T-cells product targeting the same antigen (e.g., ide-cel after cilta-cel, or vice-versa) was evaluated in some retrospective real-life series, again with inconsistent results.[58,121] In this regards, the IMWG committee consensus suggests the referral to a second anti-BCMA CAR T-cells agent only in the instance of a reasonably prolonged response to the first.[57]
Meanwhile, data on sequential anti-GPRC5D CAR T-cells after an anti-BCMA product are more compelling, with the assumption that shifting the target could counteract the occurrence of BCMA-mutated clones, as pre-clinically described.[86] In this respect, MCARH109 and arlo-cel showed promising response rates in patients previously treated with anti-BCMA CAR T, with ORR of 75-78%, despite the limitation of low numbers, and missing survival data.[27,29]
3-CAR T-cells after BsAbs therapies. CAR-T therapies preceded the introduction of BsAbs, both in clinical research and practice availability. Nevertheless, they might be used after a BsAb when the latter was given first within a clinical trial context or for more convenient availability. Altogether, prospective evidence and real-life data are scarce in this setting.
Cilta-cel was experimented in cohort C of the CARTITUDE-2 trial designed for patients previously exposed to anti-BCMA agents. As a result, patients who received previous BsAbs showed reduced ORR, mDOR and mPFS (57.1%, 8.2 mos, 5.3 mos respectively).[122] Similarly, a retrospective cohorts collected by the US Myeloma CAR T consortium focused on efficacy of ide-cel in patients previously exposed to an anti-BCMA BsAb. While ORR was 74%, comparable with other real-life observations, the 2.8 mos mPFS revealed to be undermining.[121] Interestingly, both studies showed better efficacy in terms of ORR when CAR T-cells therapies were given after prolonged amount of time from the last BsAb exposure.
On the contrary, patients who progressed under Tal within the MONUMENTAL-1 trial were showed to respond well to a subsequent anti-BCMA CAR-T agent (ORR 78%).[123] Similarly, a single center retrospective study focusing on Tal-resistant patients revealed acceptable efficacy when an anti-BCMA CAR T was given as subsequent therapy, with ORR and mPFS of 75% and 7.07 mos, respectively.[124] However, it should be reminded that both studies were limited by little numbers. In addition, a German retrospective study showed encouraging results when anti-BCMA CAR T-cells agents were given after bridging therapies with BsAbs (either Tal or Tec); on the contrary, the outcome of patients exposed to BsABs before apheresis were consistently worst, especially with prolonged exposure to teclistamab.[125]
Altogether, there is sufficient evidence to discourage anti-BCMA CAR T-cells therapy in patients progressing under BsAbs directed against the same target. The outcomes of this sequencing model predict better results when more time elapsed between the last exposure to BsAbs and CAR T-cells therapy, and when the target antigen is switched. As previously noted, data regarding BsAbs as bridging therapy after apheresis and before CAR T-cells infusion are biologically and clinically compelling.[78,79] Also, it should be reminded that the IMWG committee consensus recommends a washout period of at least 4-weeks between the last dose of BsAbs and apheretic procedures.[57]
4-Sequential BsAbs therapies. The possibility of sequencing two BsAb agents can be relevant for patients not eligible for CAR T-cells therapy and in clinical settings where access to CAR T-cells is logistically difficult.
Some evidence concerning this sequencing model emerged from the MonumelTAL-1 trial, in which 25 enrolled patients were previously exposed to a BsAb agent (23/25 anti-BCMA): the ORR was 52.2% and mDoR was 6.5 mos, both inferior as compared to patients previously exposed to BCMA-directed CAR T-cells or ADC agents. Specifically, the ORR was lower in patients who had received a BsAb as the immediate preceding line than any prior line of therapy before Tal (28.6% vs 61.1%, respectively); of note, the distance between the last dose of prior BsAb and Tal seemed to correlate with ORR.[123] Biologically, this evidence may be related to the T-cell exhaustion found in patients treated with continuous BsAb exposure, as opposed to the restoration of the T-cell repertoire when being off-therapy pressure.[105,106,126]
Some experiences of patients treated with Tal and previously exposed to anti-BCMA BsAbs are available from real-life settings. Consistently with MonumenTal-1 trial data, the ORRs reported in patients previously treated with BsAbs are inferior as compared to patients priorly exposed to CAR T-cells.[118,119]
In conclusion, the few available evidence suggests that the sequencing of two BsAbs is feasible but limited by inferior efficacy if compared to BsAbs administered after CAR T-cells therapy.[57]
Conclusions and Future Directions
The treatment landscape of RRMM has been revolutionized by the introduction of CAR T-cells and BsAbs. A learning curve regarding their best use is underway, specifically in terms of patient selection and sequencing models. In fact, it is becoming increasingly apparent that the efficacy, toxicity, and logistical profile of each drug must be properly tailored to both patients- and tumor- features, in order to maximize efficacy. Moreover, given the persisting relapsing pattern of RRMM, even when adequately controlled by TCRs, the use of sequential TCRs is common, freeing up the need for evidence-based characterization of feasible sequencing models, both in terms of safety and efficacy.[16,17,57]The current bulk of evidence enlightens that, whenever possible, the utilization of a CAR T-cell agent should be prioritized.[16,17,57] Indeed, though the initial burden on patients and health-care facilities represents a barrier to their access, CAR T-cells therapies hinder the chance of a prolonged treatment-free interval, with possible improvements in patients' quality of life. Therefore, patients should be referred to accredited centers in a timely manner, so that the manufacturing process is addressed without uncontrolled clinical progression and possibly with the chance of effective holding and bridging strategies.[16,17,57] In this regard, CAR T-cells referral should be avoided in case of rapidly progressing and clinically impactful diseases, given the well documented association of tumor burden with increased toxicities and reduced efficacy.[16,17,57] Also, judicious evaluation of patient comorbidities is necessary, given the potential burden of CRS, ICANS, prolonged cytopenias, and risk of infections, albeit real-life cohorts showed that CAR T-cells can be safely infused in patients not meeting the inclusion criteria of registrational trials in terms of comorbidities profiles, including renal impairment.[57,58,127]
On the other hand, BsAbs should be the first TCR option when dealing with features not suitable for a safe and effective referral to a CAR T-cells program. Indeed, rapidly progressing tumors might benefit from a timely initiation of therapy with off-the-shelf drugs, such as BsAbs. In addition, the reduced risk of clinically meaningful CRS, ICANS and prolonged cytopenias suggest the use of BsAbs in frail patients, though the infectious risk, the continuous schedule of administration, and the need for supportive therapies (e.g., for IVIG administration) must be carefully weighted.[16,17,57]
Of note, when patients relapse after a first TCR-exposure, treatment with another TCR agent is advised, as real-life observations have demonstrated that this is the most effective way to prolong survival.[111,112] Currently, the utilization of CAR T-cells first and then of BsAbs against a different target at the eventual relapse represents the most widely validated sequencing model within clinical trials and from real-life experiences, and should be prioritized whenever possible.[57,111-120]
Overall, the TCRs scenario is evolving rapidly, with many strands of clinical research currently underway. Allogeneic and in vivo-generated CAR T-cells are being studied, raising the chance to make this strategy a quasi-off-the-shelf one, thereby reducing the burden of manufacturing times and slots availability.[128,129] Meanwhile, fixed-durations, earlier positioning and combination strategies in BsAbs are also promising, with the intent of enhancing their efficacy while reducing toxicity profiles.[34,36,40,95,96,130,131] Moreover, dual-targeting CAR T-cells and trispecific agents (i.e., constructs capable of redirecting T-cells against two tumor antigens at once) are being investigated, with the premise of enhanced efficacy and safety.[132,133]
In addition, the anticipation of TCRs in the treatment algorithm of MM is being investigated and possibly represents the most promising evolution in the use of TCRs. Indeed, more effective and safer anti-tumor activity in contexts of reduced tumor burden and fitter T-cells repertoires is emerging from pre-clinical evidence with a solid rational, and further confirmed by clinical observations.[20,21,69,70,102] Concomitantly, randomized clinical trials are currently evaluating the use of CAR T-cells as consolidation strategies after induction phases, both in transplant-eligible and ineligible patients,[134,135] while other studies are aimed at exploring a fixed-duration schedule as a maintenance strategy with BsAbs.[136,137]
Collectively, the ever-increasing number of patients eligible for TCR therapies should come with a rationalization of health-care resources to assure these therapies as a standard-of-care treatments. Importantly, in this regards, evidence of their use in out-patient setting are being collected.[138,139] Indeed, the possibly residual tumor burden along with fixed-duration schedules are foreseen to dramatically reduce the present load of toxicities and, therefore, the need for onerous supportive therapies, thereby granting a broader access to these therapies. As a consequence, it is conceivable that the current patient selection and sequencing of therapies as we know them will progressively change, possibly eliminating the present area of uncertainty and leading to further improvement in patient outcome.
Author Contributions
MP and IS conceived the research study, performed the literature search, and wrote the original draft of the paper; KM, PT, LP, IR, MI, MT, EM, RR, SA, BT, IV supported the literature search and critically reviewed the paper; SB supported manuscript preparation, critically revised the paper, and provided editorial support; EZ conceived and supervised the research project, acquired funding, discussed the results, and critically reviewed the paper. All authors have read and accepted the final version of the manuscript.References
- Rajkumar SV. Multiple myeloma: 2024 update on
diagnosis, risk-stratification, and management. Am J Hematol. 2024
Sep;99(9):1802-24. https://doi.org/10.1002/ajh.27422 PMid:38943315
- Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024 Jan 25;390(4):301-13.
- Moreau
P, Hulin C, Perrot A, Arnulf B, Belhadj K, Benboubker L, et al.
Bortezomib, thalidomide, and dexamethasone with or without daratumumab
and followed by daratumumab maintenance or observation in
transplant-eligible newly diagnosed multiple myeloma: long-term
follow-up of the CASSIOPEIA randomised controlled phase 3 trial. Lancet
Oncol. 2024 Aug;25(8):1003-14. https://doi.org/10.1016/S1470-2045(24)00282-1 PMid:38889735
- Facon
T, Dimopoulos MA, Leleu XP, Beksac M, Pour L, Hájek R, et al.
Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple
Myeloma. N Engl J Med. 2024 Oct 31;391(17):1597-609. https://doi.org/10.1056/NEJMoa2400712 PMid:38832972
- Moreau
P, Kumar SK, Miguel JS, Davies F, Zamagni E, Bahlis N, et al. Treatment
of relapsed and refractory multiple myeloma: recommendations from the
International Myeloma Working Group. Lancet Oncol. 2021 Mar
1;22(3):e105-18.
- Gandhi
UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al.
Outcomes of patients with multiple myeloma refractory to CD38-targeted
monoclonal antibody therapy. Leukemia. 2019 Sep;33(9):2266-75. https://doi.org/10.1038/s41375-019-0435-7 PMid:30858549 PMCid:PMC6820050
- Mateos
MV, Weisel K, De Stefano V, Goldschmidt H, Delforge M, Mohty M, et al.
LocoMMotion: a prospective, non-interventional, multinational study of
real-life current standards of care in patients with relapsed and/or
refractory multiple myeloma. Leukemia. 2022 May;36(5):1371-6.
- Mateos
MV, Weisel K, De Stefano V, Goldschmidt H, Delforge M, Mohty M, et al.
LocoMMotion: a study of real-life current standards of care in
triple-class exposed patients with relapsed/refractory multiple myeloma
- 2-year follow-up (final analysis). Leukemia. 2024 Dec;38(12):2554-60.
https://doi.org/10.1038/s41375-024-02404-6 PMid:39322709 PMCid:PMC11588650
- Munshi
NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al.
Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N
Engl J Med. 2021 Feb 25;384(8):705-16. https://doi.org/10.1056/NEJMoa2024850 PMid:33626253
- Berdeja
JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al.
Ciltacabtagene autoleucel, a B-cell maturation antigen-directed
chimeric antigen receptor T-cell therapy in patients with relapsed or
refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label
study. The Lancet. 2021 Jul 24;398(10297):314-24.
- Rodriguez-Otero
P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or
Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J
Med. 2023 Mar 16;388(11):1002-14. https://doi.org/10.1056/NEJMoa2213614 PMid:36762851
- San-Miguel
J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel
or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J
Med. 2023 Jul 26;389(4):335-47. https://doi.org/10.1056/NEJMoa2303379 PMid:37272512
- Moreau
P, Garfall AL, van de Donk NWCJ, Nahi H, San-Miguel JF, Oriol A, et al.
Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med.
2022 Aug 11;387(6):495-505. https://doi.org/10.1056/NEJMoa2203478 PMid:35661166 PMCid:PMC10587778
- Lesokhin
AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et
al. Elranatamab in relapsed or refractory multiple myeloma: phase 2
MagnetisMM-3 trial results. Nat Med. 2023 Sep;29(9):2259-67. https://doi.org/10.1038/s41591-023-02528-9 PMid:37582952 PMCid:PMC10504075
- Chari
A, Minnema MC, Berdeja JG, Oriol A, Donk NWCJ van de, Rodríguez-Otero
P, et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody
for Multiple Myeloma. N Engl J Med. 2022 Dec 14;387(24):2232-44. https://doi.org/10.1056/NEJMoa2204591 PMid:36507686
- Anderson
LD, Dhakal B, Jain T, Oluwole OO, Shah GL, Sidana S, et al. Chimeric
Antigen Receptor T Cell Therapy for Myeloma: Where Are We Now and What
Is Needed to Move Chimeric Antigen Receptor T Cells Forward to Earlier
Lines of Therapy? Expert Panel Opinion from the American Society for
Transplantation and Cellular Therapy. Transplant Cell Ther. 2024
Jan;30(1):17-37. https://doi.org/10.1016/j.jtct.2023.10.022 PMid:37913909 PMCid:PMC10873054
- Mohan
M, Van Oekelen O, Akhtar OS, Cohen A, Parekh S. Charting the Course:
Sequencing Immunotherapy for Multiple Myeloma. Am Soc Clin Oncol Educ
Book Am Soc Clin Oncol Annu Meet. 2024 Jun;44(3):e432204. https://doi.org/10.1200/EDBK_432204 PMid:38875506
- Shah
UA, Mailankody S. CAR T and CAR NK cells in multiple myeloma: Expanding
the targets. Best Pract Res Clin Haematol. 2020 Mar 1;33(1):101141. https://doi.org/10.1016/j.beha.2020.101141 PMid:32139020 PMCid:PMC7137578
- Ye
B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen
receptor-T cells for cancer treatment. Mol Cancer. 2018 Feb
15;17(1):32. https://doi.org/10.1186/s12943-018-0814-0 PMid:29448937 PMCid:PMC5815249
- Ailawadhi
S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S, et al. Ide-cel vs
standard regimens in triple-class-exposed relapsed and refractory
multiple myeloma: updated KarMMa-3 analyses. Blood. 2024 Dec
5;144(23):2389-401. https://doi.org/10.1182/blood.2024024582 PMid:39197072
- Martin
T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al.
Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric
Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple
Myeloma: CARTITUDE-1 2-Year Follow-Up. J Clin Oncol. 2023 Feb
20;41(6):1265-74. https://doi.org/10.1200/JCO.22.00842 PMid:35658469 PMCid:PMC9937098
- Munshi
N, Martin T, Usmani SZ, Berdeja J, Jakubowiak A, Agha M, et al. S202:
Cartitude-1 final results: phase 1b/2 study of ciltacabtagene
autoleucel in heavily pretreated patients with relapsed/refractory
multiple myeloma. Hemasphere. 2023 Aug 8;7(Suppl):e6102468. https://doi.org/10.1097/01.HS9.0000967720.61024.68 PMCid:PMC10428265
- Mateos
MV, San-Miguel J, Dhakal B, Touzeau C, Leleu X, van de Donk NW, et al.
OA-65 Overall Survival (OS) With Ciltacabtagene Autoleucel (Cilta-cel)
Versus Standard of Care (SoC) in Lenalidomide (Len)-Refractory Multiple
Myeloma (MM): Phase 3 CARTITUDE-4 Study Update. Clin Lymphoma Myeloma
Leuk. 2024 Sep 1;24:S290 https://doi.org/10.1016/S2152-2650(24)02346-2
- Hansen
DK, Peres LC, Dima D, Richards A, Shune L, Afrough A, et al. US
Multiple Myeloma Immunotherapy Consortium. Comparison of
Standard-of-Care Idecabtagene Vicleucel and Ciltacabtagene Autoleucel
in Relapsed/Refractory Multiple Myeloma. J Clin Oncol. 2025 Feb
18:JCO2401730.
- Bishop
MR, Rosenblatt J, Dhakal B, Raje N, Cook D, Gaballa MR, et al. Phase 1
Study of Anitocabtagene Autoleucel for the Treatment of Patients with
Relapsed and/or Refractory Multiple Myeloma (RRMM): Efficacy and Safety
with 34-Month Median Follow-up. Blood. 2024 Nov 5;144(Supplement
1):4825. https://doi.org/10.1182/blood-2024-201080
- Martin
T, Raje NS, San Miguel J, Patel K, Mcloughlin L, Lui C, et al. MM-382
iMMagine-3: A Phase 3, Randomized Study to Compare the Efficacy and
Safety of Anitocabtagene Autoleucel (Anito-Cel) With Standard of Care
in Patients With Relapsed/Refractory Multiple Myeloma (RRMM). Clin
Lymphoma Myeloma Leuk. 2024. Sep1; 24: S554-5. https://doi.org/10.1016/S2152-2650(24)01676-8
- Mailankody
S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al.
GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med. 2022 Sep
28;387(13):1196-206. https://doi.org/10.1056/NEJMoa2209900 PMid:36170501 PMCid:PMC10309537
- Jurgens
EM, Firestone RS, Chaudhari J, Hosszu K, Devlin SM, Shah UA, et al.
Phase I Trial of MCARH109, a G Protein-Coupled Receptor Class C Group 5
Member D (GPRC5D)-Targeted Chimeric Antigen Receptor T-Cell Therapy for
Multiple Myeloma: An Updated Analysis. J Clin Oncol Off J Am Soc Clin
Oncol. 2024 Dec 4;JCO2401785. https://doi.org/10.1016/S2152-2650(24)01941-4
- Bal
S, Htut M, Nadeem O, Anderson LD Jr, Koçoğlu H, Gregory T, et al.
BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5
Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T-Cell
Therapy for Relapsed/Refractory Multiple Myeloma (RRMM): Updated
Results from a Phase 1 Study. Blood. 2023 Nov 2;142(Supplement 1):219. https://doi.org/10.1182/blood-2023-181857
- Bal
S, Anderson LD Jr, Nadeem O, Berdeja JG, Rossi A, Gregory T, et al.
Efficacy and Safety with Extended Follow-up in a Phase 1 Study of
BMS-986393, a G Protein-Coupled Receptor Class C Group 5 Member D
(GPRC5D)-Targeted CAR T Cell Therapy, in Patients (pts) with Heavily
Pretreated Relapsed/Refractory (RR) Multiple Myeloma (MM). Blood. 2024
Nov 5;144(Supplement 1):922. https://doi.org/10.1182/blood-2024-201356
- Bal
S, Htut M, Berdeja JG, Anderson LD Jr, Rossi A, Gregory T, et al.
BMS-986393, a G Protein-Coupled Receptor Class C Group 5 Member D
(GPRC5D)-Targeted CAR T Cell Therapy, in Patients (pts) with
Relapsed/Refractory (RR) Multiple Myeloma (MM) and 1-3 Prior Regimens:
Updated Phase 1 Safety and Efficacy Results. Blood. 2024 Nov
5;144(Supplement 1):2069. https://doi.org/10.1182/blood-2024-203279
- Velasquez
MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological
malignancies with bispecific antibodies. Blood. 2018 Jan 4;131(1):30-8.
https://doi.org/10.1182/blood-2017-06-741058 PMid:29118005 PMCid:PMC5755042
- Garfall
AL, Nooka AK, van de Donk NWCJ, Moreau P, Bhutani M, Oriol A, et al.
Long-term follow-up from the phase 1/2 MajesTEC-1 trial of teclistamab
in patients with relapsed/refractory multiple myeloma. J Clin Oncol.
2024 Jun;42(16_suppl):7540-7540. https://doi.org/10.1200/JCO.2024.42.16_suppl.7540
- Mateos
MV, Bahlis N, Costa L, Perrot A, Pei L, Rubin M, et al. PB2020:
Majestec-3: randomized, phase 3 study of Teclistamab plus Daratumumab
versus DPd or DVd in patients with relapsed/refractory multiple
myeloma. Hemasphere. 2022 Jun 23;6(Suppl):1891-1892. https://doi.org/10.1097/01.HS9.0000850912.43010.d3 PMCid:PMC9431613
- Prince
HM, Bahlis NJ, Rodríguez-Otero P, Karlin L, Akard L,
Varshavsky-Yanovsky A, et al. MagnetisMM-3: Long-Term Update and
Efficacy and Safety of Less Frequent Dosing of Elranatamab in Patients
with Relapsed or Refractory Multiple Myeloma. Blood. 2024 Nov
5;144(Supplement 1):4738. https://doi.org/10.1182/blood-2024-208192
- Leleu
X, Iida S, Ola Landgren C, Lesokhin A, Leip E, Kudla A, et al. P930:
Elranatamab monotherapy or in combination with Daratumumab vs
Daratumumab + Pomalidomide + dexamethasone for patients with
relapsed/refractory multiple myeloma: phase 3 MAGNETISMM-5 study, part
2. Hemasphere. 2023 Aug 8;7(Suppl):e052316a https://doi.org/10.1097/01.HS9.0000970624.05231.6a PMCid:PMC10431113
- Bumma
N, Richter J, Jagannath S, Lee HC, Hoffman JE, Suvannasankha A, et al.
Linvoseltamab for Treatment of Relapsed/Refractory Multiple Myeloma. J
Clin Oncol. 2024 Aug;42(22):2702-12.
- Schinke
CD, Touzeau C, Minnema MC, van de Donk NWCJ, Rodríguez-Otero P, Mateos
MV, et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a
GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple
myeloma (RRMM). J Clin Oncol. 2023 Jun;41(16_suppl):8036-8036. https://doi.org/10.1200/JCO.2023.41.16_suppl.8036
- Rasche
L, Schinke C, Touzeau C, Minnema MC, Donk NWCJ van de, Rodríguez-Otero
P, et al. MM-492 Long-term Efficacy and Safety Results From the Phase
1/2 MonumenTAL-1 Study of Talquetamab, a GPRC5D×CD3 Bispecific
Antibody, in Patients With Relapsed/Refractory Multiple Myeloma (RRMM).
Clin Lymphoma Myeloma Leuk. 2024 Sep 1;24:S561-2. https://doi.org/10.1016/S2152-2650(24)01689-6
- Cohen
YV, Moreau P, Tolbert J, Qin X, Ma X, Vieyra D, et al. MonumenTAL-3:
Phase 3 Trial of Talquetamab + Daratumumab ± Pomalidomide Versus
Daratumumab + Pomalidomide + Dexamethasone in Relapsed/Refractory
Multiple Myeloma Following ≥1 Prior Line of Therapy. Blood 2022; 140
(Supplement 1):4418-4419. https://doi.org/10.1182/blood-2022-162733
- Kumar
S, Bachier CR, Cavo M, Corradini P, Delforge M, Janowski W, et al.
CAMMA 2: A phase I/II trial evaluating the efficacy and safety of
cevostamab in patients with relapsed/refractory multiple myeloma (RRMM)
who have triple-class refractory disease and have received a prior
anti-B-cell maturation antigen (BCMA) agent. J Clin Oncol. 2023
Jun;41(16_suppl):TPS8064-TPS8064. https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS8064
- Morris
EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and
associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022
Feb;22(2):85-96. https://doi.org/10.1038/s41577-021-00547-6 PMid:34002066 PMCid:PMC8127450
- Santomasso
BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et
al. Management of Immune-Related Adverse Events in Patients Treated
With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J Clin
Oncol. 2021 Dec 10;39(35):3978-92. https://doi.org/10.1200/JCO.21.01992 PMid:34724386
- Blimark
CH, Carlson K, Day C, Einarsdottir S, Juliusson G, Karma M, et al. Risk
of infections in multiple myeloma. A populationbased study on 8,672
multiple myeloma patients diagnosed 2008-2021 from the Swedish Myeloma
Registry. Haematologica. 2025;110(1):163-72. https://doi.org/10.3324/haematol.2024.285645 PMid:39021214
- Tomasson
MH, Iida S, Niesvizky R, Mohty M, Bahlis NJ, Martinez-Lopez J, et al.
Long-term survival and safety of elranatamab in patients with relapsed
or refractory multiple myeloma: Update from the MagnetisMM-3 study.
HemaSphere. 2024;8(7):e136. https://doi.org/10.1002/hem3.136 PMid:39055646 PMCid:PMC11269363
- Lancman
G, Parsa K, Kotlarz K, Avery L, Lurie A, Lieberman-Cribbin A, et al.
IVIg Use Associated with Ten-Fold Reduction of Serious Infections in
Multiple Myeloma Patients Treated with Anti-BCMA Bispecific Antibodies.
Blood Cancer Discov. 2023 Nov 1;4(6):440-51. https://doi.org/10.1158/2643-3230.BCD-23-0049 PMid:37769148 PMCid:PMC10618720
- Hammons
L, Szabo A, Janardan A, Bhatlapenumarthi V, Annyapu E, Dhakal B, et al.
The changing spectrum of infection with BCMA and GPRC5D targeting
bispecific antibody (bsAb) therapy in patients with relapsed refractory
multiple myeloma. Haematologica. 2024;109(3):906-14. https://doi.org/10.3324/haematol.2023.283590 PMid:37646658 PMCid:PMC10905074
- Rejeski
K, Jain MD, Shah NN, Perales MA, Subklewe M. Immune effector
cell-associated haematotoxicity after CAR T-cell therapy: from
mechanism to management. Lancet Haematol. 2024 Jun;11(6):e459-70. https://doi.org/10.1016/S2352-3026(24)00077-2 PMid:38734026
- Rejeski
K, Hansen DK, Bansal R, Sesques P, Ailawadhi S, Logue JM, et al. The
CAR-HEMATOTOX score as a prognostic model of toxicity and response in
patients receiving BCMA-directed CAR-T for relapsed/refractory multiple
myeloma. J Hematol OncolJ Hematol Oncol. 2023 Jul 31;16(1):88. https://doi.org/10.1186/s13045-023-01465-x PMid:37525244 PMCid:PMC10391746
- Rejeski
K, Subklewe M, Aljurf M, Bachy E, Balduzzi A, Barba P, et al. Immune
effector cell-associated hematotoxicity: EHA/EBMT consensus grading and
best practice recommendations. Blood. 2023 Sep 7;142(10):865-77. https://doi.org/10.1182/blood.2023020578 PMid:37300386
- Hamilton
MP, Sugio T, Noordenbos T, Shi S, Bulterys PL, Liu CL, et al. Risk of
Second Tumors and T-Cell Lymphoma after CAR T-Cell Therapy. N Engl J
Med. 2024 Jun 13;390(22):2047-60. https://doi.org/10.1056/NEJMoa2401361 PMid:38865660 PMCid:PMC11338600
- Lin
Y, Qiu L, Usmani S, Joo CW, Costa L, Derman B, et al. Consensus
guidelines and recommendations for the management and response
assessment of chimeric antigen receptor T-cell therapy in clinical
practice for relapsed and refractory multiple myeloma: a report from
the International Myeloma Working Group Immunotherapy Committee. Lancet
Oncol. 2024 Aug;25(8):e374-87. https://doi.org/10.1016/S1470-2045(24)00094-9 PMid:38821074
- Rodriguez-Otero
P, Usmani S, Cohen AD, van de Donk NWCJ, Leleu X, Gállego Pérez-Larraya
J, et al. International Myeloma Working Group immunotherapy committee
consensus guidelines and recommendations for optimal use of
T-cell-engaging bispecific antibodies in multiple myeloma. Lancet
Oncol. 2024 May;25(5):e205-16. https://doi.org/10.1016/S1470-2045(24)00043-3 PMid:38697166
- Tacchetti
P, Talarico M, Barbato S, Pantani L, Mancuso K, Rizzello I, et al.
Antibody-drug conjugates, bispecific antibodies and CAR-T cells therapy
in multiple myeloma. Expert Rev Anticancer Ther. 2024 Jun;24(6):379-95.
https://doi.org/10.1080/14737140.2024.2344647 PMid:38798125
- Mateos
MV, Nooka AK, Larson SM. Moving Toward a Cure for Myeloma. Am Soc Clin
Oncol Educ Book Am Soc Clin Oncol Annu Meet. 2022 Apr;42:1-12. https://doi.org/10.1200/EDBK_349603 PMid:35623025
- Raje
N, Mateos MV, Iida S, Reece D. Clinical evidence for immune-based
strategies in early-line multiple myeloma: current challenges in
decision-making for subsequent therapy. Blood Cancer J. 2023 Mar
22;13(1):41. https://doi.org/10.1038/s41408-023-00804-y PMid:36944635 PMCid:PMC10030780
- Costa
LJ, Banerjee R, Mian H, Weisel K, Bal S, Derman BA, et al.
International myeloma working group immunotherapy committee
recommendation on sequencing immunotherapy for treatment of multiple
myeloma. Leukemia. 2025 Jan 27; https://doi.org/10.1038/s41375-024-02482-6 PMid:39870767 PMCid:PMC11879857
- Sidana
S, Patel KK, Peres LC, Bansal R, Kocoglu MH, Shune L, et al. Safety and
efficacy of standard-of-care ciltacabtagene autoleucel for
relapsed/refractory multiple myeloma. Blood. 2025 Jan 2;145(1):85-97. https://doi.org/10.1182/blood.2024025945 PMid:39365257
- Hansen
DK, Sidana S, Peres LC, Colin Leitzinger C, Shune L, Shrewsbury A, et
al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma:
Real-World Experience From the Myeloma CAR T Consortium. J Clin Oncol
Off J Am Soc Clin Oncol. 2023 Apr 10;41(11):2087-97. https://doi.org/10.1200/JCO.22.01365 PMid:36623248 PMCid:PMC10082273
- Ferment
B, Lambert J, Caillot D, Lafon I, Karlin L, Lazareth A, et al. French
early nationwide idecabtagene vicleucel chimeric antigen receptor
T-cell therapy experience in patients with relapsed/refractory multiple
myeloma (FENIX): A real-world IFM study from the DESCAR-T registry. Br
J Haematol. 2024 Sep;205(3):990-8. https://doi.org/10.1111/bjh.19505 PMid:38747092
- Shah
N, Munshi NC, Berdeja JG, Jagannath S, Finney O, Martin N, et al.
Baseline Correlates of Complete Response to Idecabtagene Vicleucel
(ide-cel, bb2121), a BCMA-Directed CAR T Cell Therapy in Patients with
Relapsed and Refractory Multiple Myeloma: Subanalysis of the KarMMa
Trial. Blood. 2021 Nov 5;138(Supplement 1):1739. https://doi.org/10.1182/blood-2021-148375
- Raje
NS, Siegel DS, Jagannath S, Lonial S, Munshi NC, Moreau P, et al.
Idecabtagene Vicleucel (ide-cel, bb2121) in Relapsed and Refractory
Multiple Myeloma: Analyses of High-Risk Subgroups in the KarMMa Study.
Blood. 2020 Nov 5;136(Supplement 1):37-8. https://doi.org/10.1182/blood-2020-134319
- Cohen
AD, Parekh S, Santomasso BD, Gállego Pérez-Larraya J, van de Donk NWCJ,
Arnulf B, et al. Incidence and management of CAR-T neurotoxicity in
patients with multiple myeloma treated with ciltacabtagene autoleucel
in CARTITUDE studies. Blood Cancer J. 2022 Feb 24;12(2):32. https://doi.org/10.1038/s41408-022-00629-1 PMid:35210399 PMCid:PMC8873238
- Logue
JM, Peres LC, Hashmi H, Colin-Leitzinger CM, Shrewsbury AM, Hosoya H,
et al. Early cytopenias and infections after standard of care
idecabtagene vicleucel in relapsed or refractory multiple myeloma.
Blood Adv. 2022 Dec 27;6(24):6109-19. https://doi.org/10.1182/bloodadvances.2022008320 PMid:35939783 PMCid:PMC9768247
- Ikegawa
S, Sperling AS, Ansuinelli M, Nikiforow S, Quinn D, Bu D, et al.
T-ChargeTM Manufacturing of the Anti-BCMA CAR-T, Durcabtagene
Autoleucel (PHE885), Promotes Expansion and Persistence of CAR-T Cells
with High TCR Repertoire Diversity. Blood. 2023 Nov 2;142(Supplement
1):3469. https://doi.org/10.1182/blood-2023-177721
- Ravi
G, Richard S, Kumar S, Atrash S, Liedtke M, Kaur G, et al. Phase 1
clinical trial of B-Cell Maturation Antigen (BCMA) NEX-T® Chimeric
Antigen Receptor (CAR) T cell therapy CC-98633/BMS-986354 in
participants with triple-class exposed multiple myeloma. Leukemia. 2025
Feb 5; https://doi.org/10.1038/s41375-025-02518-5 PMid:39910285 PMCid:PMC11976278
- Oliver-Caldés
A, González-Calle V, Cabañas V, Español-Rego M, Rodríguez-Otero P,
Reguera JL, et al. Fractionated initial infusion and booster dose of
ARI0002h, a humanised, BCMA-directed CAR T-cell therapy, for patients
with relapsed or refractory multiple myeloma (CARTBCMA-HCB-01): a
single-arm, multicentre, academic pilot study. Lancet Oncol. 2023
Aug;24(8):913-24. https://doi.org/10.1016/S1470-2045(23)00222-X PMid:37414060
- Iacoboni
G, Navarro V, Martín-López AÁ, Rejeski K, Kwon M, Jalowiec KA, et al.
Recent Bendamustine Treatment Before Apheresis Has a Negative Impact on
Outcomes in Patients With Large B-Cell Lymphoma Receiving Chimeric
Antigen Receptor T-Cell Therapy. J Clin Oncol Off J Am Soc Clin Oncol.
2024 Jan 10;42(2):205-17. https://doi.org/10.1200/JCO.23.01097 PMid:37874957
- Garfall
AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, et al. T-cell
phenotypes associated with effective CAR T-cell therapy in
postinduction vs relapsed multiple myeloma. Blood Adv. 2019 Oct
8;3(19):2812-5. https://doi.org/10.1182/bloodadvances.2019000600 PMid:31575532 PMCid:PMC6784521
- Cohen
AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et
al. B cell maturation antigen-specific CAR T cells are clinically
active in multiple myeloma. J Clin Invest. 2019 Mar 21;129(6):2210-21. https://doi.org/10.1172/JCI126397 PMid:30896447 PMCid:PMC6546468
- Gandhi
AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory
agents lenalidomide and pomalidomide co-stimulate T cells by inducing
degradation of T cell repressors Ikaros and Aiolos via modulation of
the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014
Mar;164(6):811-21.
- Works
M, Soni N, Hauskins C, Sierra C, Baturevych A, Jones JC, et al.
Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T cell
Function against Multiple Myeloma Is Enhanced in the Presence of
Lenalidomide. Mol Cancer Ther. 2019 Dec;18(12):2246-57. https://doi.org/10.1158/1535-7163.MCT-18-1146 PMid:31395689
- Aleman
A, Grossman L, Kogan Zajdman A, Chen LY, Reci S, Jagannath S, et al.
Improving Anti-BCMA CAR-T Functionality with Novel Celmods in Multiple
Myeloma. Blood. 2024 Nov 5;144(Supplement 1):3259. https://doi.org/10.1182/blood-2024-201186
- Meermeier
EW, Sharik M, Du M, Stein CK, Zhu YX, Shi CX, et al.
Celmod-Dexamethasone Combination Overcomes Primary Resistance to T Cell
Engagers Caused By High Tumor Burden in a Human CRBN+ Preclinical Model
of Multiple Myeloma. Blood. 2024 Nov 5;144(Supplement 1):356. https://doi.org/10.1182/blood-2024-212225
- Zafar
A, Huang CY, Lo M, Arora S, Chung A, Wong SW, et al. Intensity of
Cyclophosphamide-Based Bridging Therapy Before Chimeric Antigen
Receptor T Cell Therapy in Myeloma. Transplant Cell Ther. 2023 Aug
1;29(8):504.e1-504.e7. https://doi.org/10.1016/j.jtct.2023.05.016 PMid:37244643
- Afrough
A, Hashmi H, Hansen DK, Sidana S, Ahn C, Peres LC, et al. Real-world
impact of bridging therapy on outcomes of ide-cel for myeloma in the
U.S. Myeloma Immunotherapy Consortium. Blood Cancer J. 2024 Apr
12;14(1):63. https://doi.org/10.1038/s41408-024-00993-0 PMid:38609386 PMCid:PMC11015040
- Anguille
S, Leleu X, Gatt M, Karlin L, Spencer A, Iida S, et al. P-005
Effectiveness of Bridging Therapy Corresponds to Improved Outcomes
After Receiving CAR-T Therapy: Phase 3 CARTITUDE-4 Study of Patients
With Relapsed, Lenalidomide-Refractory Multiple Myeloma. Clin Lymphoma
Myeloma Leuk. 2024 Sep 1;24:S42-3. https://doi.org/10.1016/S2152-2650(24)01908-6
- Dhakal
B, Akhtar OS, Cowan AJ, Richard S, Friend R, Rees MJ, et al.
Talquetamab Bridging: Paving the Way to B-Cell Maturation Antigen
(BCMA) CAR-T Cell Therapy in Relapsed/Refractory Multiple Myeloma
(RRMM). Blood. 2024 Nov 5;144(Supplement 1):931. https://doi.org/10.1182/blood-2024-202017
- Fandrei
D, Seiffert S, Rade M, Rieprecht S, Gagelmann N, Born P, et al.
Bispecific Antibodies as Bridging to BCMA CAR-T Cell Therapy for
Relapsed/Refractory Multiple Myeloma. Blood Cancer Discov. 2025 Jan
8;6(1):38-54. https://doi.org/10.1158/2643-3230.BCD-24-0118 PMid:39441177
- Akhtar
OS, Hashmi H, Oloyede T, Brazauskas R, Bye M, Sidana S, et al. Real
World Outcomes of Older Adults and Frail Patients with
Relapse/Refractory Multiple Myeloma Receiving Idecabtagene Vicleucel.
Transplant Cell Ther Off Publ Am Soc Transplant Cell Ther. 2024 Feb
1;30(2):S184-5. https://doi.org/10.1016/j.jtct.2023.12.239
- Modi
K, Akhtar OS, Al-Jumayli M, Extermann M, Booth-Jones M, De Avila G, et
al. Association of frailty and high-risk immuno-nutritional score with
outcomes in patients with relapsed and refractory multiple myeloma
treated with chimeric antigen receptor T-cells. J Clin Oncol. 2023
Jun;41(16_suppl):12052-12052. https://doi.org/10.1200/JCO.2023.41.16_suppl.12052
- Reynolds
G, Cliff ERS, Mohyuddin GR, Popat R, Midha S, Liet Hing MN, et al.
Infections following bispecific antibodies in myeloma: a systematic
review and meta-analysis. Blood Adv. 2023 Oct 10;7(19):5898-903. https://doi.org/10.1182/bloodadvances.2023010539 PMid:37467036 PMCid:PMC10558589
- Delforge
M, Shah N, Miguel JSF, Braverman J, Dhanda DS, Shi L, et al.
Health-related quality of life with idecabtagene vicleucel in relapsed
and refractory multiple myeloma. Blood Adv. 2022 Feb 22;6(4):1309-18. https://doi.org/10.1182/bloodadvances.2021005913 PMid:34933328 PMCid:PMC8864645
- Martin
T, Lin Y, Agha M, Cohen AD, Htut M, Stewart AK, et al. Health-related
quality of life in patients given ciltacabtagene autoleucel for
relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b-2,
open-label study. Lancet Haematol. 2022 Dec;9(12):e897-905. https://doi.org/10.1016/S2352-3026(22)00284-8 PMid:36215989
- Ferreri
CJ. CARving a Path Forward: Unveiling T Cell Phenotypes Across the
Spectrum of Multiple Myeloma Disease Progression to Inform Future
Strategies for Overcoming Resistance to CAR T Therapy. Transplant Cell
Ther. 2024 Feb;30(2):126-8. https://doi.org/10.1016/j.jtct.2024.01.058 PMid:38307689
- Lee
H, Neri P, Bahlis NJ. BCMA- or GPRC5D-targeting bispecific antibodies
in multiple myeloma: efficacy, safety, and resistance mechanisms.
Blood. 2024 Mar 28;143(13):1211-7. https://doi.org/10.1182/blood.2023022499 PMid:38194680
- Martin
TG, Moreau P, Usmani SZ, Garfall A, Mateos MV, San-Miguel JF, et al.
Teclistamab Improves Patient-Reported Symptoms and Health-Related
Quality of Life in Relapsed or Refractory Multiple Myeloma: Results
From the Phase II MajesTEC-1 Study. Clin Lymphoma Myeloma Leuk. 2024
Mar;24(3):194-202. https://doi.org/10.1016/j.clml.2023.11.001 PMid:38052709
- Mohty
M, Bahlis NJ, Nooka AK, DiBonaventura M, Ren J, Conte U. Impact of
elranatamab on quality of life: Patient-reported outcomes from
MagnetisMM-3. Br J Haematol. 2024 May;204(5):1801-10. https://doi.org/10.1111/bjh.19346 PMid:38420657
- Schinke
C, Touzeau C, Oriol A, Mateos MV, Stevens DA, Rasche L, et al.
Symptoms, Functioning, and Health-Related Quality of Life in Patients
with Relapsed/Refractory Multiple Myeloma Treated with Talquetamab:
Updated Patient-Reported Outcomes from the Phase 1/2 MonumenTAL-1
Study. Blood. 2023 Nov 2;142(Supplement 1):6711. https://doi.org/10.1182/blood-2023-189151
- Rasche L, Hudecek M, Einsele H. CAR T-cell therapy in multiple myeloma: mission accomplished? Blood. 2024 Jan 25;143(4):305-10. https://doi.org/10.1182/blood.2023021221 PMid:38033289
- García-Guerrero
E, Sierro-Martínez B, Pérez-Simón JA. Overcoming Chimeric Antigen
Receptor (CAR) Modified T-Cell Therapy Limitations in Multiple Myeloma.
Front Immunol. 2020 Jun 5;11:1128. https://doi.org/10.3389/fimmu.2020.01128 PMid:32582204 PMCid:PMC7290012
- Zhou
X, Kortuem KM, Rasche L, Einsele H. Bispecific antibody and chimeric
antigen receptor (CAR) modified T-cell in the treatment of multiple
myeloma: Where do we stand today? Presse Medicale Paris Fr 1983. 2024
Dec 9;54(1):104265. https://doi.org/10.1016/j.lpm.2024.104265 PMid:39662761
- Arnulf
B, Kerre T, Agha ME, Delforge M, Braunschweig I, Shah N, et al.
Efficacy and safety of ciltacabtagene autoleucel ± lenalidomide
maintenance in newly diagnosed multiple myeloma with suboptimal
response to frontline autologous stem cell transplant: CARTITUDE-2
cohort D. J Clin Oncol. 2024 Jun;42(16_suppl):7505-7505. https://doi.org/10.1200/JCO.2024.42.16_suppl.7505
- Usmani
S, Patel K, Hari P, Berdeja J, Alsina M, Vij R, et al. KarMMa-2 Cohort
2a: Efficacy and Safety of Idecabtagene Vicleucel in Clinical High-Risk
Multiple Myeloma Patients with Early Relapse after Frontline Autologous
Stem Cell Transplantation. Blood. 2022 Nov 15;140(Supplement 1):875-7. https://doi.org/10.1182/blood-2022-162469
- Cohen
YC, Magen H, Gatt M, Sebag M, Kim K, Min CK, et al. Talquetamab plus
Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med.
2025 Jan 9;392(2):138-49. https://doi.org/10.1056/NEJMoa2406536 PMid:39778168
- Dholaria
BR, Weisel K, Mateos MV, Goldschmidt H, Martin TG, Morillo D, et al.
Talquetamab (tal) + daratumumab (dara) in patients (pts) with
relapsed/refractory multiple myeloma (RRMM): Updated TRIMM-2 results. J
Clin Oncol. 2023 Jun;41(16_suppl):8003-8003. https://doi.org/10.1200/JCO.2023.41.16_suppl.8003
- Dhodapkar
MV. Immune status and selection of patients for immunotherapy in
myeloma: a proposal. Blood Adv. 2024 May 28;8(10):2424-32. https://doi.org/10.1182/bloodadvances.2023011242 PMid:38564776 PMCid:PMC11112605
- Bahlis
NJ, Costello CL, Raje NS, Levy MY, Dholaria B, Solh M, et al.
Elranatamab in relapsed or refractory multiple myeloma: the
MagnetisMM-1 phase 1 trial. Nat Med. 2023 Oct;29(10):2570-6. https://doi.org/10.1038/s41591-023-02589-w PMid:37783970 PMCid:PMC10579053
- Hashmi
H, Hansen DK, Peres LC, Puglianini OC, Freeman C, De Avila G, et al.
Factors associated with refractoriness or early progression after
idecabtagene vicleucel in patients with relapsed/ refractory multiple
myeloma: US Myeloma Immunotherapy Consortium real world experience.
Haematologica. 2024 May 1;109(5):1514-1524. https://doi.org/10.3324/haematol.2023.283888
- Dhodapkar
KM, Cohen AD, Kaushal A, Garfall AL, Manalo RJ, Carr AR, et al. Changes
in Bone Marrow Tumor and Immune Cells Correlate with Durability of
Remissions Following BCMA CAR T Therapy in Myeloma. Blood Cancer
Discov. 2022 Nov 2;3(6):490-501. https://doi.org/10.1158/2643-3230.BCD-22-0018 PMid:36026513 PMCid:PMC9627239
- Leblay
N, Maity R, Hasan F, Neri P. Deregulation of Adaptive T Cell Immunity
in Multiple Myeloma: Insights Into Mechanisms and Therapeutic
Opportunities. Front Oncol. 2020;10:636. https://doi.org/10.3389/fonc.2020.00636 PMid:32432039 PMCid:PMC7214816
- Neri
P, Ahn S, Lee H, Leblay N, Friedrich M, Maity R, et al. Dysfunctional
Hyper-Expanded Clonotypes and Lack of TCR Clonal Replacement Predict
Resistance to T Cell Engagers in Multiple Myeloma. Blood. 2022 Nov
15;140(Supplement 1):2093-4. https://doi.org/10.1182/blood-2022-164717
- Lin
Y, Raje NS, Berdeja JG, Siegel DS, Jagannath S, Madduri D, et al.
Idecabtagene vicleucel for relapsed and refractory multiple myeloma:
post hoc 18-month follow-up of a phase 1 trial. Nat Med. 2023
Sep;29(9):2286-94. https://doi.org/10.1038/s41591-023-02496-0 PMid:37592106 PMCid:PMC10504071
- Montes
de Oca R, Gu J, Zhao H, Zelinsky K, Wu D, Davis C, et al. Biomarker
Correlates of Response to Ciltacabtagene Autoleucel in Patients with
Relapsed or Refractory Multiple Myeloma from CARTITUDE-1, a Phase 1b/2
Open-Label Study, at the ~3 Year Follow-up. Blood. 2023 Nov 2;142:2099.
https://doi.org/10.1182/blood-2023-182298
- Philipp
N, Kazerani M, Nicholls A, Vick B, Wulf J, Straub T, et al. T-cell
exhaustion induced by continuous bispecific molecule exposure is
ameliorated by treatment-free intervals. Blood. 2022 Sep
8;140(10):1104-18. https://doi.org/10.1182/blood.2022015956 PMid:35878001 PMCid:PMC10652962
- Chakraborty
R, Cheruvalath H, Patwari A, Szabo A, Schinke C, Dhakal B, et al.
Sustained remission following finite duration bispecific antibody
therapy in patients with relapsed/refractory myeloma. Blood Cancer J.
2024 Aug 12;14(1):137. https://doi.org/10.1038/s41408-024-01114-7 PMid:39134535 PMCid:PMC11319778
- Lesokhin
AM, Richter J, Trudel S, Cohen AD, Spencer A, Forsberg PA, et al.
Enduring Responses after 1-Year, Fixed-Duration Cevostamab Therapy in
Patients with Relapsed/Refractory Multiple Myeloma: Early Experience
from a Phase I Study. Blood. 2022 Nov 15;140(Supplement 1):4415-7. https://doi.org/10.1182/blood-2022-157547
- Lee
H, Ahn S, Maity R, Leblay N, Ziccheddu B, Truger M, et al. Mechanisms
of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in
multiple myeloma. Nat Med. 2023 Sep;29(9):2295-306. https://doi.org/10.1038/s41591-023-02491-5 PMid:37653344 PMCid:PMC10504087
- Anderson,
Jr LD, Munshi NC, Shah N, Jagannath S, Berdeja JG, Lonial S, et al.
Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell
therapy, in relapsed and refractory multiple myeloma: Updated KarMMa
results. J Clin Oncol. 2021 May 20;39(15_suppl):8016-8016. https://doi.org/10.1200/JCO.2021.39.15_suppl.8016
- Papadimitriou
M, Ahn S, Diamond B, Lee H, McIntyre J, Truger M, et al. Timing
antigenic escape in multiple myeloma treated with T-cell redirecting
immunotherapies. BioRxiv Prepr Serv Biol. 2024 May
26;2024.05.22.595383. https://doi.org/10.1101/2024.05.22.595383
- Van
Oekelen O, Nath K, Mouhieddine TH, Farzana T, Aleman A, Melnekoff DT,
et al. Interventions and outcomes of patients with multiple myeloma
receiving salvage therapy after BCMA-directed CAR T therapy. Blood.
2023 Feb 16;141(7):756-65. https://doi.org/10.1182/blood.2022017848 PMid:36327160 PMCid:PMC10082354
- Mouhieddine
TH, Van Oekelen O, Melnekoff DT, Li J, Ghodke-Puranik Y, Lancman G, et
al. Sequencing T-cell redirection therapies leads to deep and durable
responses in patients with relapsed/refractory myeloma. Blood Adv. 2023
Mar 28;7(6):1056-64. https://doi.org/10.1182/bloodadvances.2022007923 PMid:36018226 PMCid:PMC10033902
- Touzeau
C, Krishnan AY, Moreau P, Perrot A, Usmani SZ, Manier S, et al.
Efficacy and safety of teclistamab in patients with relapsed/refractory
multiple myeloma after BCMA-targeting therapies. Blood. 2024 Dec
5;144(23):2375-88. https://doi.org/10.1182/blood.2023023616 PMid:39172760
- Nooka
AK, Lesokhin AM, Mohty M, Niesvizky R, Maisel C, Arnulf B, et al.
Efficacy and safety of elranatamab in patients with relapsed/refractory
multiple myeloma (RRMM) and prior B-cell maturation antigen
(BCMA)-directed therapies: A pooled analysis from MagnetisMM studies. J
Clin Oncol. 2023 Jun;41(16_suppl):8008-8008. https://doi.org/10.1200/JCO.2023.41.16_suppl.8008
- Riedhammer
C, Bassermann F, Besemer B, Bewarder M, Brunner F, Carpinteiro A, et
al. Real-world analysis of teclistamab in 123 RRMM patients from
Germany. Leukemia. 2024 Feb;38(2):365-71. https://doi.org/10.1038/s41375-024-02154-5 PMid:38245601 PMCid:PMC10844072
- Reyes
KR, Liu YC, Huang CY, Banerjee R, Martin T, Wong SW, et al. Salvage
therapies including retreatment with BCMA-directed approaches after
BCMA CAR-T relapses for multiple myeloma. Blood Adv. 2024 May
14;8(9):2207-16. https://doi.org/10.1182/bloodadvances.2023012066 PMid:38429087 PMCid:PMC11061209
- Jakubowiak
AJ, Anguille S, Karlin L, Chari A, Schinke C, Rasche L, et al. Updated
Results of Talquetamab, a GPRC5D×CD3 Bispecific Antibody, in Patients
with Relapsed/Refractory Multiple Myeloma with Prior Exposure to T-Cell
Redirecting Therapies: Results of the Phase 1/2 MonumenTAL-1 Study.
Blood. 2023 Nov 2;142:3377. https://doi.org/10.1182/blood-2023-187242
- Shaikh
H, Lochner J, Loeffler B, Friend R, Habib A, McKenna M, et al.
Talquetamab in B Cell Maturation Antigen (BCMA) Exposed
Relapsed-Refractory Multiple Myeloma Patients. Blood. 2024 Nov
5;144(Supplement 1):5170.4. https://doi.org/10.1182/blood-2024-211018
- Castaneda
O, Hansen DK, Vazquez-Martinez MA, Modukuri S, Blue B, Fadul J, et al.
Effect of Talquetamab on Responses in Patients with Relapsed and
Refractory Multiple Myeloma with Prior Exposure to T-Cell Redirecting
Therapies. Blood. 2024 Nov 5;144(Supplement 1):5168. https://doi.org/10.1182/blood-2024-211739
- Kumar
S, Berdeja J, Sborov D, Corradini C, Cohen YC, Lesokhin A, et al.
Cevostamab in patients with RRMM who are triple-class refractory and
have received a prior BCMA-targeted ADC or CAR T-cell: initial results
from the Phase I/II CAMMA 2 study. HemaSphere. 2024 Jun
- Ferreri
CJ, Hildebrandt MAT, Hashmi H, Shune LO, McGuirk JP, Sborov DW, et al.
Real-world experience of patients with multiple myeloma receiving
ide-cel after a prior BCMA-targeted therapy. Blood Cancer J. 2023 Aug
9;13(1):1-9. https://doi.org/10.1038/s41408-023-00886-8 PMid:37558706 PMCid:PMC10412575
- Cohen
AD, Mateos MV, Cohen YC, Rodriguez-Otero P, Paiva B, van de Donk NWCJ,
et al. Efficacy and safety of cilta-cel in patients with progressive
multiple myeloma after exposure to other BCMA-targeting agents. Blood.
2023 Jan 19;141(3):219-30. https://doi.org/10.1182/blood.2022015526 PMid:36095849 PMCid:PMC10562529
- Sanchez
L, Schinke C, Krishnan A, Berdeja JG, van de Donk NW, Mateos MV, et al.
Clinical Outcomes of Subsequent Therapies in Patients with
Relapsed/Refractory Multiple Myeloma Following Talquetamab Treatment:
Analyses from the Phase 1/2 MonumenTAL-1 Study. Blood. 2023 Nov
2;142(Supplement 1):2007. https://doi.org/10.1182/blood-2023-182330
- Joiner
L, Costa L, Giri S, Ravi G, Godby K, Hagedorn C, et al. P-036 BCMA
after GPRC5D: Efficacy of BCMA-Directed Therapy on Patients with
Relapsed/Refractory Multiple Myeloma (RRMM) Progressing After
GPRC5D-Directed Therapy. Clin Lymphoma Myeloma Leuk. 2024 Sep
1;24:S61-2. https://doi.org/10.1016/S2152-2650(24)01939-6
- Waldschmidt
J, Fandrei D, Vucinic V, Wäsch R, Nogai A, Alsdorf W, et al. Efficacy
of Anti-BCMA CAR-T Cell Therapies in Multiple Myeloma Patients with
Prior Exposure to Bispecific Antibodies- Results from a Retrospective
Multi-Center Registry Analysis. Blood. 2024 Nov 5;144(Supplement
1):2007. https://doi.org/10.1182/blood-2024-210451
- Verkleij
CPM, O'Neill CA, Broekmans MEC, Frerichs KA, Bruins WSC, Duetz C, et
al. T-Cell Characteristics Impact Response and Resistance to
T-Cell-Redirecting Bispecific Antibodies in Multiple Myeloma. Clin
Cancer Res Off J Am Assoc Cancer Res. 2024 Jul 15;30(14):3006-22. https://doi.org/10.1158/1078-0432.CCR-23-3333 PMid:38687588
- Wäsch
R, Strüssmann T, Wehr C, Marks R, Meyer PT, Walz G, et al. Safe and
successful CAR T-cell therapy targeting BCMA in a multiple myeloma
patient requiring hemodialysis. Ann Hematol. 2023 May;102(5):1269-70. https://doi.org/10.1007/s00277-023-05163-z PMid:36930259 PMCid:PMC10102086
- Dholaria
B, Kocoglu M, Andrew K, Ramakrishnan A, Shune L, Ganguly S, et al.
OA-04 A Phase 1 Study of P-BCMA- ALLO1, a Non-viral, Allogeneic BCMA
Directed CAR-T in Relapsed/Refractory Multiple Myeloma (RRMM). Clin
Lymphoma Myeloma Leuk. 2024 Sep 1;24:S3. https://doi.org/10.1016/S2152-2650(24)01845-7
- Mullard A. In vivo CAR T cells move into clinical trials. Nat Rev Drug Discov. 2024 Oct;23(10):727-30. https://doi.org/10.1038/d41573-024-00150-z PMid:39284972
- Razzo
B, Grady C, Susanibar-Adaniya S, Waxman A, Vogl DT, Cohen AD, et al. A
Phase 2, Single-Arm, Non-Inferiority Study of Limited-Duration
Teclistamab for Relapsed and Refractory Multiple Myeloma (LimiTec).
Blood 2023; 142 (Supplement 1): 3394. https://doi.org/10.1182/blood-2023-177735
- Harrison
S, Riley CH, Manier S, Yoon SS, Pinto A, Cazaubiel T, et al. OA-05
Efficacy of forimtamig, a GPRC5DxCD3 bispecific antibody, in patients
with relapsed/refractory multiple myeloma (RRMM): analysis of patient
and disease-related factors associated with responses. Clin Lymphoma
Myeloma Leuk. 2023 Sep 1;23:S3-4. https://doi.org/10.1016/S2152-2650(23)01572-0
- Du
J, Fu WJ, Jiang H, Dong B, Gao L, Liu L, et al. Updated results of a
phase I, open-label study of BCMA/CD19 dual-targeting fast CAR-T GC012F
for patients with relapsed/refractory multiple myeloma (RRMM). J Clin
Oncol. 2023 Jun;41(16_suppl):8005-8005. https://doi.org/10.1200/JCO.2023.41.16_suppl.8005
- van
de Donk NWCJ, O'Neill C, de Ruijter MEM, Verkleij CPM, Zweegman S.
T-cell redirecting bispecific and trispecific antibodies in multiple
myeloma beyond BCMA. Curr Opin Oncol. 2023 Nov 1;35(6):601-11. https://doi.org/10.1097/CCO.0000000000000983 PMid:37501530 PMCid:PMC10566598
- Dytfeld
D, Dhakal B, Agha M, Manier S, Delforge M, Kuppens S, et al.
Bortezomib, Lenalidomide and Dexamethasone (VRd) Followed By
Ciltacabtagene Autoleucel Versus Vrd Followed By Lenalidomide and
Dexamethasone (Rd) Maintenance in Patients with Newly Diagnosed
Multiple Myeloma Not Intended for Transplant: A Randomized, Phase 3
Study (CARTITUDE-5). Blood. 2021 Nov 23;138:1835. https://doi.org/10.1182/blood-2021-146210
- Broijl
A, San-Miguel J, Suzuki K, Krishnan A, van de Donk N, Cook G, et al.
P23 Emagine/cartitude-6: a randomized phase 3 study of dvrd followed by
ciltacabtagene autoleucel versus dvrd followed by autologous stem cell
transplant in transplant-eligible patients with newly diagnosed
multiple myeloma. HemaSphere. 2023 May 9;7(Suppl):22-3. https://doi.org/10.1097/01.HS9.0000936220.68605.8b PMCid:PMC10171573
- Zamagni
E, Silzle T, Špička I, Tahri S, Lonergan S, Nijhof IS, et al. Phase 3
Study of Teclistamab (Tec) in Combination with Lenalidomide (Len) and
Tec Alone Versus Len Alone in Newly Diagnosed Multiple Myeloma (NDMM)
As Maintenance Therapy Following Autologous Stem Cell Transplantation
(ASCT): Safety Run-in (SRI) Results from the Majestec-4/EMN30 Trial.
Blood. 2024 Nov 5;144(Supplement 1):494. https://doi.org/10.1182/blood-2024-205608
- Mateos
Manteca MV, Grosicki S, Kim K, Negre E, Vandendries E. MagnetisMM-7: An
open-label, multicenter, randomized phase 3 study of elranatamab versus
lenalidomide in post-transplant patients with newly diagnosed multiple
myeloma. J Clin Oncol. 2023 Jun;41(16_suppl):TPS8066-TPS8066. https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS8066
- Furqan
F, Bhatlapenumarthi V, Dhakal B, Fenske TS, Farrukh F, Longo W, et al.
Outpatient administration of CAR T-cell therapies using a strategy of
no remote monitoring and early CRS intervention. Blood Adv. 2024 Aug
15;8(16):4320-9. https://doi.org/10.1182/bloodadvances.2024013239 PMid:38889435 PMCid:PMC11372811
- Sandahl
TB, Soefje SA, Fonseca R, Ailawadhi S, Parrondo R, Lin D, et al.
Real-World Safety and Health Care Resource Utilization of Teclistamab
Under an Outpatient Model for Step-Up Dosing Administration. JCO Oncol
Pract. 2024 Dec;0(0):OP-24-00489. https://doi.org/10.1200/OP-24-00489 PMid:39705632