Historically, the sequencing of salvage therapies was relatively simple, mainly revolving around the use of a combination of proteasome inhibitors and immunomodulatory agents (IMiDs), mostly lenalidomide, usually combined with corticosteroids. However, with the advent of monoclonal antibodies, drug conjugate antibodies (ADC), bispecific antibodies (Bs), and CAR-T cell therapies, together with the increasing number of novel agents (Selinexor, Melflufen) and combinations, the approach to treatment relapse and sequencing has become more and more challenging. Overall, recent updates highlight the importance of tailoring therapy based on the following factors:
- Prior therapy exposure. A key principle is, when possible, to switch the class of therapeutic agents. At the present time, in Italy, the first-line treatment for the large majority of newly diagnosed transplant-eligible (NDTE) MM patients is represented by the combination of Daratumumab - Bortezomib - Thalidomide - Dexamethasone (D-VTD) followed by autologous stem cell transplantation (ASCT) and lenalidomide maintenance, whereas for most of transplant-ineligible (NTE) patients, the first line therapy is represented by the triplet of Daratumumab-Lenalidomide-Dexamethasone (D-RD).
- Depth of response to prior therapies. Some patients may have had prolonged periods of remission with prior therapies, while others experience rapid progressive disease. The prognosis of patients with short-lasting remissions (usually below 18 months) or refractory disease is unfavorable, and these patients may benefit from more aggressive treatments, possibly including new immunotherapy approaches (such as CAR-T or Bispecific antibodies), which offer new avenues of treatment when traditional options have failed.
- Patients’ conditions. Frailty and associated comorbidities might strongly impact the choice of available therapies.
Sequencing of Myeloma treatment after Lenalidomide refractoriness
Lenalidomide (Len) is nowadays the most used immunomodulatory drug in the treatment of MM, and it plays a pivotal role in the management of both newly diagnosed and relapsed/refractory MM. However, with the widespread use of Len in the first line of treatment both in TE and NTE patients, a significant proportion of patients eventually become refractory to Len at first relapse.[1,2] While still inquiring of whether progression on low dose Len (10 mg used in maintenance) was consistent with progression to full dose (25 mg), retrospective studies indicate the lack of relationship between the outcomes of Len-resistant patients and Len doses during progression, relapse at lower doses (5-15 mg) still being associated with poor outcome.[3] After a patient becomes refractory to Len, the treatment plan should focus on drugs with different mechanisms of action or capable of overcoming the mechanism of resistance. The most common approach in the Len-refractory setting is to use combination therapies, which have been shown to increase response rates compared to single-agent therapy. The most relevant combinations are discussed below (Figure 1 and Table 1).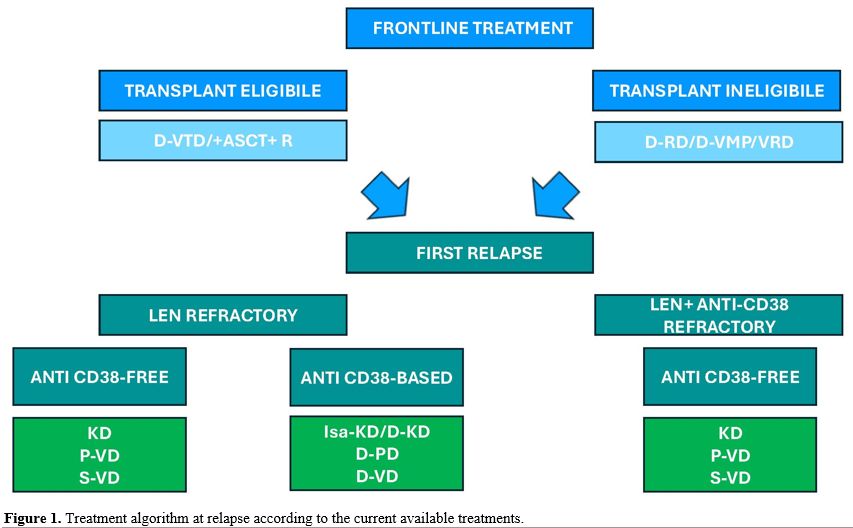 |
Figure 1. Treatment algorithm at relapse according to the current available treatments. |
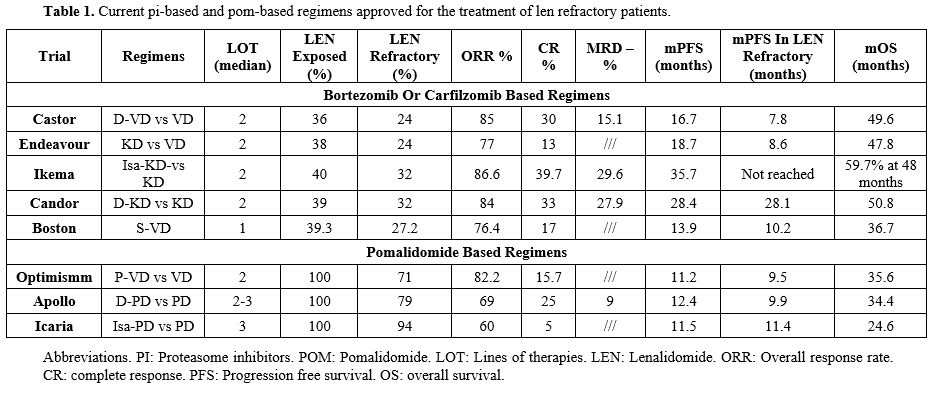 |
Table 1. Current pi-based and pom-based regimens approved for the treatment of len refractory patients. |
Anti-CD38 plus Carfilzomib and Dexamethasone combinations. The IKEMA and CANDOR trials both demonstrated that the addition of the anti-CD38 monoclonal antibody (Isatuximab or Daratumumab, respectively) to Carfilzomib and Dexamethasone significantly improved overall response rate (ORR), minimal residual disease (MRD) negativity rates and progression-free survival (PFS 35.7 and 28.4 months, ORR 87% and 84%, > VGPR 73% and 69%, MRD negativity rates 33.5% and 18%, respectively), with an improvement in overall survival (OS) compared to Carfilzomib and Dexamethasone alone.[4-9] Although not pre-specified in the protocols, the same results were also reported for Len refractory patients, accounting for roughly 30% of enrolled patients in both trials. Concerning safety, more patients in the anti-CD38 arms complain of hematological toxicities (mostly neutropenia and thrombocytopenia) and nonhematological (mostly upper respiratory tract infections), although treatment discontinuations were consistent with the control group. Interestingly a recently published paper, 103 real-life patients treated with IsaKD showed consistent results with respect to the original trial; interestingly, nearly all patients were len-exposed (19%) and Len‐refractory (71%), thus reinforcing the data of IKEMA.[10]
Anti-CD38 plus Pomalidomide and Dexamethasone combinations. The APOLLO study demonstrated that the combination of Daratumumab-Pomalidomide-Dexamethasone (D-PD) significantly improves PFS (12.4 months) and ORR (69%) in patients with RR MM, compared to Pomalidomide and Dexamethasone alone. In this phase 3 trial, 89% of patients were Len refractory, with a median number of previous lines of two. This combination also granted deeper responses (≥ VGPR 51%), with a 9% rate of MRD negativity, and showed a manageable safety profile.[11,12] Additionally, the phase 2 MM014 trial evaluated the D-PD regimen in 112 patients after 1 or 2 previous lines of therapy and prior Len exposition, with 75% of cases Len refractory. With a median follow-up of 28.4 months, Len refractory patients showed an ORR of 76.2%, with 47.6% achieving a VGPR or better and PFS of 23.7 months, with no new safety signals observed.[13]
Interestingly, a real-life experience of D-PD recently published reported a mPFS of 18.9 months in patients progressing on Len and receiving second-line D-PD, thus reinforcing the MM014 results.[14]
The EMA approved the combinations of Pomalidomide and Dexamethasone with Isatuximab (Isa-PD) in the context of RRMM patients with 2 previous lines of therapy (LOT). Specifically, 307 patients, almost all patients (94%) were refractory to Len, who had received at least two previous LOTs, were randomized to receive Isa-Pd (154) or Pd (153) in the ICARIA trial.[15] With a median follow-up of 35.3 months, PFS was 11.1 vs 5.9 months in the control arm (p<0·0001), and higher ORR and depth of response were reported.[16,17] Though treatment discontinuation was infrequent, dose reductions were more frequent in the Isa-Pd arm, with pneumonia being the most frequent serious AE (all grades, both groups: 23% for Isa-Pd and 21% in controls).[18] Of note, with a follow-up to approximately 52 months, Isa-Pd maintained a significant advantage with a benefit of 6.9-month improvement in OS (24.6 vs 17.7 months), proving Isa-Pd to be an option for patients who are refractory to other treatments, such as Len.[18,19]
Pomalidomide Bortezomib and Dexamethasone (P-VD). This combination was evaluated in the phase 3 OPTIMISMM trial, which was designed to assess the efficacy and safety of P-VD compared to VD in patients with RRMM who had received one to three prior lines of therapy. The results of this study showed that the median PFS was longer with P-VD (11.2 months), with a 39% reduction in the risk of disease progression or death compared to Vd. At first relapse, Len refractory patients showed a PFS of 17.8 months, with an advantage in ORR (85.9%), quality of response (>VGPR 56.3%), and MRD negativity rates. Concerning safety, neutropenia (35.9% vs. 12.9%) and thrombocytopenia (17.2% vs. 22.6%), peripheral sensory neuropathy (9.4% vs. 3.2%) and infections (29.7% vs 21.0%) were the most common grade 3/4 hematologic and nonhematologic TEAEs (P-VD vs VD), respectively.[20-22]
Daratumumab Bortezomib and Dexamethasone (D-VD) and Carfilzomib Dexamethasone (KD). In the CASTOR and the ENDEAVOUR trials, which evaluated the D-VD and KD, respectively, a clear PFS advantage was obtained with respect to the VD control arm, particularly at the first relapse (D-VD 27 months and KD 22.2 months); however, considering Len refractory patients (accounting for 24% of the study population in each trial), median PFS was 7.8 and 8.6 months, respectively. Consistently, both D-VD and KD yielded better PFS when used at first relapse; however, the advantage over VD in len refractory patients is markedly less pronounced.[23-28]
Selinexor – Bortezomib - Dexamethasone (S-VD). Selinexor is an oral, selective inhibitor of nuclear export (SINE) that blocks XPO1 (exportin 1), a protein responsible for transporting various tumor suppressor proteins and other important proteins out of the nucleus. The Selinexor-Bortezomib-Dexamethasone combination (S-VD) has been evaluated in the BOSTON trial, where S-VD demonstrated a significant PFS advantage with respect to VD.[29] However, the number of patients previously exposed to Dara was low (6%) and only 37% of patients were previously exposed to Len.[29] Interestingly, post hoc analysis from the BOSTON trial with 28 months follow-up further supported the role of S-VD in patients with Len-refractory disease (26% of total, n=106) at first relapse, particularly if PI naïve, showing a significant improvement in OS (OS 26.7 vs 18.6 months, respectively; HR 0.53; p = 0.015) compared with VD. The median PFS was longer in all subgroups (Len refractory: 10.2 vs. 7.1 months, PI-naïve: 29.5 vs. 9.7; bortezomib-naïve: 29.5 vs. 9.7; 1LOT: 21.0 vs. 10.7; p < .05), highlighting the importance of a double class switch in these patients.[30] One of the major concerns with Selinexor is represented by its toxicity profile, which includes hematologic toxicities (e.g., thrombocytopenia, neutropenia) and nonhematologic toxicities (e.g., fatigue, nausea, anorexia, and gastrointestinal effects), often leading to discontinuation (median time to discontinuation in Len-refractory patients: 6.1 vs 4.7 months for S-VD and VD arms, respectively), raising questions about tolerability of this combination. These effects were mitigated by dose modification of combination therapies, frequent monitoring of blood counts, and supportive care. Pre-medication with anti-nausea agents and adjusting the dose of dexamethasone may also help manage some side effects. These adverse events (AE) were also the most represented in a recently published real-world analysis.[31] Of notice, the once-weekly bortezomib administration significantly reduced the peripheral neuropathy rates with respect to bi-weekly administration scheduled in other regimens (i.e. D-VD or P-VD).
Novel Belantamab-based combinations. Belantamab mafodotin (Bela) is a BCMA-targeted antibody-drug conjugate (ADC) that has emerged as a promising treatment option in RRMM, particularly in patients who have become refractory to Len and Dara. Bela is composed of an anti-BCMA monoclonal antibody conjugated to a cytotoxic drug, monomethyl auristatin F (MMAF), which is delivered directly to the BCMA-expressing plasma cells. Once bound to BCMA, the ADC is internalized, and the cytotoxic drug MMAF induces cell death, making it an effective therapeutic agent in the treatment of multiple myeloma. The presence of BCMA on malignant plasma cells and its critical role in the survival of these cells makes BCMA-targeted therapies a central focus in the treatment of MM, especially for patients who have already undergone multiple lines of therapy.
In the DREAMM-2 study, single-agent belantamab mafodotin showed a response rate of about 31% in heavily pretreated patients, including those who were triple and penta-class refractory, and the treatment was associated with durable responses. In this setting, corneal toxicity (keratopathy) remains a significant dose-limiting side effect that has led to the investigation of combination therapies to optimize outcomes and minimize adverse effects.[32,33] Clinical trials investigating this combination have shown very encouraging results, with bortezomib potentially mitigating some of the immune suppression that can occur with daratumumab and lenalidomide resistance.
In the DREAMM-7 trial, Bela in combination with VD (Bela-VD) has been evaluated in 494 RRMM patients after at least one prior line of therapy, 34% of whom were Len-refractory (53% Len-exposed). In this study, after a median follow-up of 28.2 months, although the ORR was not clearly different from the control arm Dara-VD (82.7% vs. 71.3%, respectively), the PFS (36.6 vs. 13.4 months, respectively), the percentage of MRD negativity (MRD negativity 38.7% vs 17.1%) and DOR (35.6 vs 17.8 months) were significantly improved.[34] Notably, though OS was not reached in either arm at first interim analysis, OS at 18 months was 84% in the Bela-VD group and 73% in the Dara-VD group, with a strong benefit in favor of Bela-VD (HR:0.57).[34] Interestingly, the advantage observed with Bela was extended to all subgroups, including Len refractory patients, that achieved an mPFS of 25 months and CR plus MRD of 25% compared to 6% with Dara-VD).
At the same time, Bela, in combination with Pomalidomide and Dexamethasone (Bela-PD), was tested in the DREAMM-8 trial. This study included 100% of Len-exposed patients with ¼ of previously anti-CD38 mAb exposure. At a median follow-up of 21.8 months, the results are extremely interesting, with a 48% reduction of risk of progression or death and a superior PFS compared to the control arm P-VD (12.7 months in the P-VD group), with a 12-month estimated PFS of 71% with Bela-Pd and 51% with P-VD. ORR was 77% (40% ≥CR, 24% MRD negative) vs 72% (16% ≥CR, 5% MRD negative), while follow-up for OS is ongoing.[35] Of note, among patients treated in the second line (n=82 in the Bela-arm, n=77 in the P-VD arm), PFS was not reached with Bela-Pd and 18.5 months with P-VD (12-month PFS rate: 78% vs. 64%; ≥CR: 46% vs. 23%, with MRD negativity of 27% vs 4%; median DOR: not reached vs 18.0 months, respectively). Specifically, results from Len-refractory patients receiving 2-line treatment (66 and 53 patients in the two arms, respectively) mPFS was not reached with Bela-Pd and 13.1 months with P-VD (12-month PFS rate: 74% vs. 53%; ≥CR: 44% vs. 21%, with MRD negativity of 22% vs 3%; median DOR: not reached vs 13.8 months; respectively).[36] AE is, as expected, mostly ocular (43% grade 3 or 4) but was effectively managed by protocol-recommended dose modification of Bela and was characterized by high rates of resolution, low treatment discontinuation rates, and the absence of a negative effect on patient-assessed QoL.
Sequencing of Multiple Myeloma Therapy After anti-CD38 mAb and Lenalidomide Refractoriness
The daratumumab-lenalidomide-dexamethasone (D-RD) regimen represents the first choice for NTE NDMM patients, significantly improving their survival outcomes.[37] However, a substantial proportion of patients are becoming both Dara and Len refractory, posing a complex challenge for clinicians. In line with this data, although at the time of this review, the number of Dara and Len refractory/relapsed patients is relatively low (the combination was introduced at relapse in Italy in 2018 and for the first line in 2021), it is expected that the number of double refractory patients will increase. Roughly 3,000 RRMM patients will become Dara and Len refractory in the period 2024-2028. Thus, refractoriness to these agents represents a critical point in disease progression. According to guidelines, actual treatment strategies at first relapse following Dara Len include PI-based and/or Pomalidomide-based combination, to which has been recently added the combination of S-VD (Figure 2 and Table 2). The potential utility of anti-CD38 mAb retreatment does not represent an appealing opportunity since preliminary results showed no significant benefit.[38]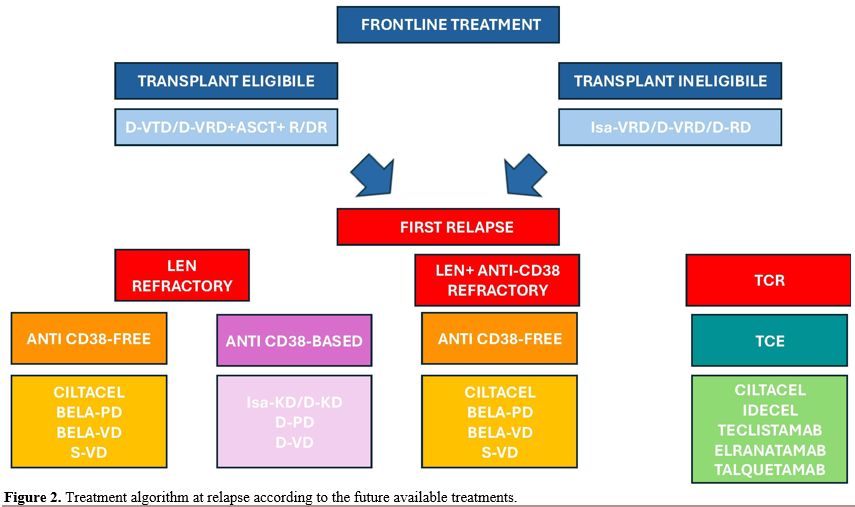 |
Figure 2. Treatment algorithm at relapse according to the future available treatments. |
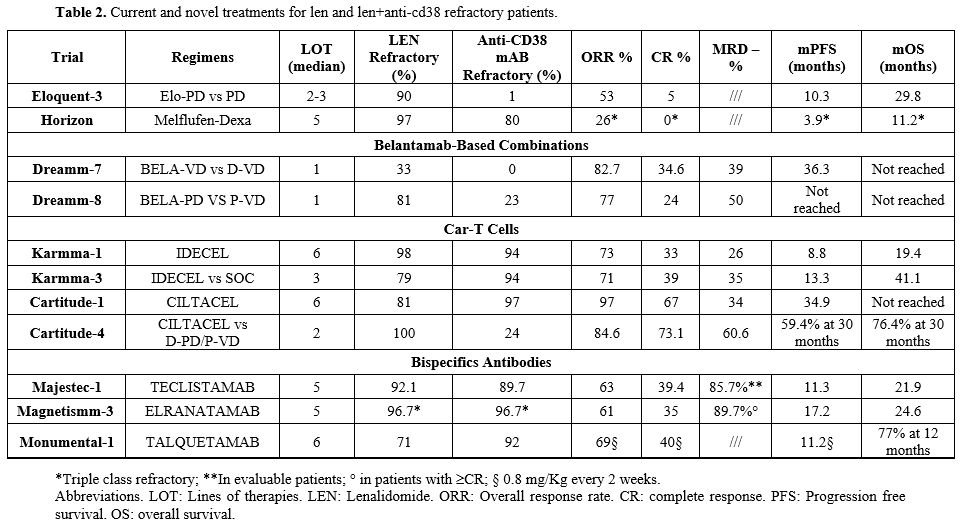 |
Table 2. Current and novel treatments for len and len+anti-cd38 refractory patients. |
The use of KD and P-VD has already been discussed in previous paragraphs. In CD38 and Len refractory patients, the combination of Elotuzumab with Pomalidomide and Dexamethasone (Elo-PD) after at least two lines of therapy has demonstrated efficacy (PFS 10.3 months and OS 29.8 months) with a low-level of toxicity in a phase 2 trial.[39,40] However, this study did not include patients with anti-CD38 mAb treatment, and an Italian real-life experience has reported a significantly lower response to this combination in Dara-refractory patients.[41,42]
Melphalan sulfenamide (Melflufen) is a recently approved chemotherapy drug in the treatment of MM, according to data from the Ocean trial, namely in patients with relapsed/refractory multiple myeloma who have not received an ASCT or progressed >36 months after receiving an ASCT.[43,44] It is a hybrid molecule that combines the alkylating agent melphalan with a peptide linker, allowing for more targeted delivery of the drug to myeloma cells. Melflufen is an alkylating agent that works by binding to the DNA of cancer cells, leading to cross-linking of DNA strands, preventing replication, and triggering cell death. Ongoing trials and studies have shown that melflufen when used with dexamethasone, provides promising results (PFS 5.6 months and OS 23.4 months) for patients who are heavily pretreated (i.e., who have undergone at least three previous lines of prior therapy). Side effects and toxicity concerns have been raised. One of the most common adverse effects is hematologic toxicity, particularly thrombocytopenia and neutropenia.[43,44]
As previously reported, the combinations of Bela-VD and Bela-PD represent an interesting option for these patients. In particular, Bela-PD represents a promising combination in this setting since in the DREAMM-8 study, the percentages of Len and anti-CD38 mAb refractory patients were 81% and 23%, respectively. Although these therapies will represent a relevant improvement in the RRMM setting, novel immunotherapies have favored the more relevant progress in the therapy of RRMM. These approaches include CAR-T cells and Bispecific Antibodies.
CAR-T cell therapy
Idecabtagene vicleucel (Ide-cel) is the first class anti-BCMA CAR-T cell therapy approved due to the results of the KarMMa-1 trial, which enrolled patients with six previous lines of therapy, some patients being still in remission after 3 years follow up.[45] Real-life results were consistent with those of the clinical trial, although more than 70% of patients treated in real life did not meet the criteria for the KarMMA-1 study.[46] More recently, in the KarMMa-3 trial enrolling Triple Class–Exposed (TCE) RRMM patients who have received at least two prior therapies, ide-cel compared to P-VD, obtained an extended PFS (PFS: 13.3 vs. 4.4 months, P <0.001), ORR (71% vs. 42%, p<0.0001) including ≥CR (44% vs 6%) and ≥CR plus MRD-negativity (35% vs 2%, at 10-5 sensitivity) and OS (41.4 vs 37.9 months), with an overall manageable toxicity profile.[47,48] Of great interest, Ide-cel has demonstrated a favorable risk-to-benefit ratio in the KarMMa-2 cohorts 2a and 2c in early relapse of the disease with high rate, deep and sustained responses in patients with RRMM progressing within 18 months after treatment initiation, with a manageable safety profile.[49,50]More appealing results originate from the second approved anti-BCMA CAR-T cell, Ciltacabtagene autoleucel (Cilta-cel). The CARTITUDE 1 study enrolled patients with more than six previous lines of therapy, 87% of whom were triple-class refractory and 40% penta-refractory. The ORR was nearly 98%, with an MRD negativity rate of 90% (for valuable patients), the PFS was 34.7 months, and the median OS was not reached.[51,52]
The CARTITUDE-4 is a phase 3 clinical study designed to assess the efficacy and safety of Cilta-cel in Len refractory patients with 1-3 prior lines of treatment. The CARTITUDE-4 study has shown enthusiastic results, with ORR of 85% and many patients achieving stringent complete responses (CR >73%) and a median PFS that has not been reached and is significantly higher with respect to the control arm (either D-PD or P-VD, PFS 11 months). At the last updated at 30 months follow up, PFS in the Cilta-cel and control arms was 59.4% and 25.7%, respectively (HR (95% CI): 0.29 (0.22–0.39); P<0.0001), while 30-month OS was 76.4% vs 63.8% in the groups, respectively (HR, 0.55; 95% CI 0.39-0.79; P = 0.0009).[53,54]
While CAR-T cells showed promising efficacy in Len and Dara refractory patients, there are notable safety concerns associated with these therapies that require careful management. Both Ide-cel and Cilta-cel have been associated with cytokine-release syndrome (CRS), though generally manageable with supportive care and the use of IL-6 inhibitors like tocilizumab. Interestingly, in the CARTITUDE-4 trial, CRS was reported to have a lower incidence with respect to the registration study, likely due to a better bridging therapy, and, in most cases, it was mild to moderate. Immune effector cell–associated neurotoxicity syndrome (ICANS) is another known side effect of CAR-T therapies; fortunately, neurotoxicity with Cilta-cel in the CARTITUDE-4 trial appears to be manageable, and most cases are reversible. CAR-T cells are also associated with hematologic toxicities, such as cytopenias (e.g., neutropenia, thrombocytopenia, and anemia), which are common after the infusion due to the impact on the bone marrow. Prolonged cytopenias can occur, and patients may require growth factors and transfusions to support blood counts during recovery. Due to the immunosuppressive effects of CAR-T therapy and the pre-conditioning chemotherapy, infections are a concern. Prophylactic measures for bacterial, viral, and fungal infections are typically recommended during the initial months following CAR-T cell infusion.
Bispecific Antibodies in Refractory Multiple Myeloma. Another promising approach to treat RRMM is the use of bispecific antibodies (BsAbs). These are engineered antibodies that can bind simultaneously to two different antigens. In the context of multiple myeloma, bispecific antibodies are designed to engage both the CD3 receptor on T cells and tumor-associated antigens (such as BCMA, GPRC5D, or FCRH5) on the surface of myeloma cells. This dual targeting facilitates T-cell activation and directs them to kill the myeloma cells, leading to improved anti-tumor activity. Up to now, the anti-BCMA BsAbs Teclistamab and Elranatamab and the anti-GPRC5D BsAb Talquetamab are available for the treatment of RRMM.
Teclistamab activity was investigated in the Majestec-1 trial. In this study, Cohort A included patients with a median of 5 previous lines of therapies (LOT), nearly 60% of triple class refractory (TCR), and 30% of penta refractory (PCR). At 30 months of follow-up, the ORR is 63% (≥ VGPR 59.4%), the median PFS is 11.3 months, and the median OS is 21.9 months. Major toxicities were represented by neutropenia and infection (the latter 50% of grade 3-4), whereas CRS and ICANS incidence was very low and mostly of grade 1-2.[55] Real-life data on Teclistamab were recently published by American and European groups, with consistent results. In these studies, the large majority of patients would have been excluded from the Majestec-1 trial, mostly for previous exposition to anti-BCMA agents, either CAR-T cells or Belantamab Mafodotin. However, efficacy data were consistent with the pivotal trial.[56]
Elranatamab efficacy and toxicities were evaluated in the MagnetisMM-3 trial. In this study, patients included presented a median LOT of 5, and half of them were PCR. The recently updated results reported an ORR of 61% (≥ VGPR nearly 60%) and median PFS and OS of 17.2 months and 24.6 months, respectively. As for Teclistamab, major toxicities were represented by neutropenia and infections, while CRS, although reported in 71% of cases, were mostly of grades 1-2. Finally, ICANS was less than 4%. Overall, the observed safety profile with Elranatamab is consistent with that of Teclistamab as well as other BCMA‐targeted bispecific antibodies.[57,58]
Talquetamab, the first-in-class BsAb targeting GPRC5D, was explored in the MonumeTAL-1 study, which included heavily pretreated patients (median LOT=6, TCR=75%, PCR=25%). Despite a different target, the results were almost superimposable with the other BsAb, with ORR of 60-70% and a median PFS of 7.5 and 11.9 months in the once-weekly and every-two-week dosing cohorts, respectively.[59]
Current present and future directions in Salvage Therapy Sequencing
Although the sequencing of therapy in RRMM is evolving rapidly, the current standard for Len refractory patients at first relapse typically involves IsaKD or D-PD in patients who are naïve to or still sensitive to anti-CD38 monoclonal antibodies. For others, combinations like S-VD, P-VD, or KD can offer viable alternatives. The choice between these options is often influenced by factors such as the patient's overall fitness, cardiovascular health, and the need for biweekly intravenous administration of carfilzomib. On the other hand, Dara-PD, which benefits from the subcutaneous administration of daratumumab, may influence the decision for third-line therapy. In patients who are double-refractory to Daratumumab and Lenalidomide, S-VD and, in the coming years, Bela-VD are considered the most appropriate options.Additionally, CAR-T therapies and BsAbs are poised to play an increasingly crucial role. Cilta-cel, in particular, has shown promising results in Len refractory patients at first relapse, as demonstrated in the CARTITUDE-4 trial, offering significant benefits over current standard therapies. However, concerns remain regarding the real-world feasibility of these therapies, especially as potential second-line options. One of the major issues is the optimal sequencing between CAR-T and BsAb. Real-world evidence suggests that anti-BCMA CAR-T should be offered before anti-BCMA BsAb, while Talquetamab could be an appropriate bridging therapy to Idecel or Ciltacel.[60,61] The high number of patients in need of CAR-T therapy and the limited number of Centers capable of administering these treatments pose substantial challenges. Moreover, the requirement for dedicated hospital beds at each Center represents a logistical barrier. Bispecific antibodies, which are off-the-shelf treatments, may soon offer a more feasible alternative to CAR-T, yet robust data on the optimal sequencing to maximize their efficacy is still needed. The lower risk of severe infections compared to anti-BCMA BsAb, along with the efficacy demonstrated even after previous anti-BCMA therapy, could be key factors in the choice of Talquetamab in patients with reduced performance status or with a high risk of infectious complications. Finally, the management of ongoing therapy and treatment-related toxicities remains critical when using these agents.
Although not likely available soon, another interesting class of drugs, i.e., the cereblon E3 ligase modulator (Celmod) compounds, characterized by increased potency, selectivity for cereblon, and enhanced immune stimulation compared to IMiDs, would contribute to improving the possibility of limiting the neoplastic plasm cell growth. Iberdomide and Mezigdomide were specifically designed to achieve rapid, potent, and deep degradation of Ikaros and Aiolos, key transcription factors in hematopoietic cell development and differentiation, and initial promising data have been recently published with the use of these drugs in heavily pretreated MM patients.
Furthermore, the sequencing of salvage therapies will likely be influenced by several key factors.[64] Biomarker-driven therapies, particularly as mechanisms of resistance to treatment become better understood, will play an increasingly important role. As the molecular profiling of multiple myeloma advances, it will become clearer which patients would benefit most from specific therapies, such as BCL-2 inhibitors or BCMA-targeted therapies. The challenge of optimizing treatment combinations will also intensify in the near future. The most effective ways to combine newer drug classes (e.g., CAR-T, BsAbs) with traditional agents (e.g., proteasome inhibitors, IMiDs, Celmod) are still being explored in clinical trials, with the goal of improving response rates and overall survival. Another promising avenue is the combination of different BsAbs (e.g., Talquetamab and Teclistamab), which have shown encouraging results in difficult-to-treat conditions like extramedullary disease, even if careful attention must be given to severe adverse events related to these drugs. Finally, ongoing trials of new therapies - such as novel monoclonal antibodies targeting alternative immune checkpoints, small molecule inhibitors, and next-generation CAR-T therapies - are set to expand the therapeutic options available for salvage treatments. These developments will provide additional sequencing choices for patients with relapsed/refractory disease.
Conclusions
The sequencing of salvage therapies in multiple myeloma is a dynamic and evolving process influenced by the increasing number of available therapeutic agents. Personalization based on the patient's disease characteristics, prior treatment history, and individual preferences is central to optimizing outcomes. As new drugs and strategies emerge, clinicians must continuously update their treatment algorithms to ensure that patients with RRMM receive the most effective and appropriate therapies at each stage of disease progression. The future of salvage therapy sequencing in multiple myeloma holds promise, with the potential for even more tailored and efficacious treatments.References
- Kumar S, Paiva B, Anderson KC, Durie B,
Landgren O, Moreau P, Munshi N, Lonial S, Blade J, Mateos MV,
Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H,
Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath
S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG,
McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S,
Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau
H. International Myeloma Working Group consensus criteria for response
and minimal residual disease assessment in multiple myeloma. Lancet
Oncol. 2016;17:e328-e46. https://doi.org/10.1016/S1470-2045(16)30206-6 PMid:27511158
- Rajkumar
SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J,
Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau
H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi
NC, San Miguel J, International Myeloma Workshop Consensus P. Consensus
recommendations for the uniform reporting of clinical trials: report of
the International Myeloma Workshop Consensus Panel 1. Blood.
2011;117:4691-5. https://doi.org/10.1182/blood-2010-10-299487 PMid:21292775 PMCid:PMC3710442
- Kastritis E, Terpos E, Dimopoulos MA. How I treat relapsed multiple myeloma. Blood. 2022;139:2904-17. https://doi.org/10.1182/blood.2020008734 PMid:35007326
- Moreau
P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Spicka
I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y,
Suzuki K, Risse ML, Asset G, Mace S, Martin T, group Is. Isatuximab,
carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a
multicentre, open-label, randomised phase 3 trial. Lancet.
2021;397:2361-71. https://doi.org/10.1016/S0140-6736(21)00592-4 PMid:34097854
- Martin
T, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Spicka
I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y,
Suzuki K, Casca F, Mace S, Risse ML, Moreau P. Isatuximab, carfilzomib,
and dexamethasone in patients with relapsed multiple myeloma: updated
results from IKEMA, a randomized Phase 3 study. Blood Cancer J.
2023;13:72. https://doi.org/10.1038/s41408-023-00797-8 PMid:37156782 PMCid:PMC10166682
- Yong
K, Martin T, Dimopoulos MA, Mikhael J, Capra M, Facon T, Hajek R,
Spicka I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A,
Koh Y, Suzuki K, Casca F, Mace S, Risse ML, Moreau P. Isatuximab plus
carfilzomib-dexamethasone versus carfilzomib-dexamethasone in patients
with relapsed multiple myeloma (IKEMA): overall survival analysis of a
phase 3, randomised, controlled trial. Lancet Haematol.
2024;11:e741-e50. https://doi.org/10.1016/S2352-3026(24)00148-0 PMid:39067465
- Dimopoulos
M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Yang H,
Klippel Z, Zahlten-Kumeli A, Usmani SZ. Carfilzomib, dexamethasone, and
daratumumab versus carfilzomib and dexamethasone for patients with
relapsed or refractory multiple myeloma (CANDOR): results from a
randomised, multicentre, open-label, phase 3 study. Lancet.
2020;396:186-97. https://doi.org/10.1016/S0140-6736(20)30734-0 PMid:32682484
- Usmani
SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K,
Gavriatopoulou M, Oriol A, Rabin N, Nooka A, Qi M, Beksac M, Jakubowiak
A, Ding B, Zahlten-Kumeli A, Yusuf A, Dimopoulos M. Carfilzomib,
dexamethasone, and daratumumab versus carfilzomib and dexamethasone for
patients with relapsed or refractory multiple myeloma (CANDOR): updated
outcomes from a randomised, multicentre, open-label, phase 3 study.
Lancet Oncol. 2022;23:65-76. https://doi.org/10.1016/S1470-2045(21)00579-9 PMid:34871550
- Usmani
SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Shu X,
Li C, Dimopoulos M. Final analysis of carfilzomib, dexamethasone, and
daratumumab vs carfilzomib and dexamethasone in the CANDOR study. Blood
Adv. 2023;7:3739-48. https://doi.org/10.1182/bloodadvances.2023010026 PMid:37163358 PMCid:PMC10368773
- De
Novellis D, Derudas D, Vincelli D, Fontana R, Della Pepa R, Palmieri S,
Accardi F, Rotondo F, Morelli E, Gigliotta E, Roccotelli D, Marano L,
Barone ML, Cetani G, Esposito D, Lazzaro A, Delle Cave G, Serio B,
Morini D, Porrazzo M, Urciuoli E, Masucci C, Fanelli F, Rizzo M,
Arcamone M, Trastulli F, Rocco S, Leone A, Bianco R, Salvatore F, Idato
A, Sicari M, Tosi P, Rascato MG, Di Perna M, Falcone AP, Morello L,
Carlisi M, Svanera G, Annunziata M, Frigeri F, Califano C, Carella AM,
Marcacci G, Pane F, Risitano AM, Giudice V, Botta C, Selleri C.
Clinical Efficacy of Isatuximab Plus Carfilzomib and Dexamethasone in
Relapsed/Refractory Multiple Myeloma Patients. Eur J Haematol.
2025;114:105-14. https://doi.org/10.1111/ejh.14314 PMid:39370303 PMCid:PMC11613624
- Dimopoulos
MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, Moreau
P, Baldini L, Symeonidis A, Bila J, Oriol A, Mateos MV, Einsele H,
Orfanidis I, Ahmadi T, Ukropec J, Kampfenkel T, Schecter JM, Qiu Y,
Amin H, Vermeulen J, Carson R, Sonneveld P, Investigators AT.
Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and
dexamethasone alone in previously treated multiple myeloma (APOLLO): an
open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:801-12. https://doi.org/10.1016/S1470-2045(21)00128-5 PMid:34087126
- Dimopoulos
MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, Moreau
P, Baldini L, Symeonidis A, Bila J, Oriol A, Mateos MV, Einsele H,
Orfanidis I, Kampfenkel T, Liu W, Wang J, Kosh M, Tran N, Carson R,
Sonneveld P. Subcutaneous daratumumab plus pomalidomide and
dexamethasone versus pomalidomide and dexamethasone in patients with
relapsed or refractory multiple myeloma (APOLLO): extended follow up of
an open-label, randomised, multicentre, phase 3 trial. Lancet Haematol.
2023;10:e813-e24. https://doi.org/10.1016/S2352-3026(23)00218-1 PMid:37793772
- Bahlis
NJ, Samaras C, Reece D, Sebag M, Matous J, Berdeja JG, Shustik J,
Schiller GJ, Ganguly S, Song K, Seet CS, Acosta-Rivera M, Bar M, Quick
D, Fonseca G, Liu H, Gentili C, Singh P, Siegel D.
Pomalidomide/Daratumumab/Dexamethasone in Relapsed or Refractory
Multiple Myeloma: Final Overall Survival From MM-014. Clin Lymphoma
Myeloma Leuk. 2024;24:852-62. https://doi.org/10.1016/j.clml.2024.07.014 PMid:39237427
- Mian
H, Eisfeld C, Venner CP, Masih-Khan E, Kardjadj M, Jimenez-Zepeda VH,
Khandanpour C, Lenz G, McCurdy A, Sebag M, Song K, LeBlanc R, White D,
Stakiw J, Reiman A, Louzada M, Aslam M, Kotb R, Gul E, Reece D.
Efficacy of Daratumumab-Containing Regimens Among Patients With
Multiple Myeloma Progressing on Lenalidomide Maintenance: Retrospective
Analysis. Front Oncol. 2022;12:826342. https://doi.org/10.3389/fonc.2022.826342 PMid:35251992 PMCid:PMC8894582
- Attal
M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, Leleu
X, Schjesvold F, Moreau P, Dimopoulos MA, Huang JS, Minarik J, Cavo M,
Prince HM, Mace S, Corzo KP, Campana F, Le-Guennec S, Dubin F, Anderson
KC, group I-Ms. Isatuximab plus pomalidomide and low-dose dexamethasone
versus pomalidomide and low-dose dexamethasone in patients with
relapsed and refractory multiple myeloma (ICARIA-MM): a randomised,
multicentre, open-label, phase 3 study. Lancet. 2019;394:2096-107. https://doi.org/10.1016/S0140-6736(19)32556-5 PMid:31735560
- Richardson
PG, Perrot A, San-Miguel J, Beksac M, Spicka I, Leleu X, Schjesvold F,
Moreau P, Dimopoulos MA, Huang JS, Minarik J, Cavo M, Prince HM,
Malinge L, Dubin F, van de Velde H, Anderson KC. Isatuximab plus
pomalidomide and low-dose dexamethasone versus pomalidomide and
low-dose dexamethasone in patients with relapsed and refractory
multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase
3 study. Lancet Oncol. 2022;23:416-27. https://doi.org/10.1016/S1470-2045(22)00019-5 PMid:35151415
- Bringhen
S, Pour L, Vorobyev V, Vural F, Warzocha K, Benboubker L, Koh Y,
Maisnar V, Karlin L, Pavic M, Campana F, Le Guennec S, Menas F, van de
Velde H, Richardson PG. Isatuximab plus pomalidomide and dexamethasone
in patients with relapsed/refractory multiple myeloma according to
prior lines of treatment and refractory status: ICARIA-MM subgroup
analysis. Leuk Res. 2021;104:106576. https://doi.org/10.1016/j.leukres.2021.106576 PMid:33839618
- Richardson
PG, Perrot A, Miguel JS, Beksac M, Spicka I, Leleu X, Schjesvold F,
Moreau P, Dimopoulos MA, Huang SY, Minarik J, Cavo M, Prince HM, Mace
S, Zhang R, Dubin F, Morisse MC, Anderson KC.
Isatuximab-pomalidomide-dexamethasone versus pomalidomide-dexamethasone
in patients with relapsed and refractory multiple myeloma: final
overall survival analysis. Haematologica. 2024;109:2239-49. https://doi.org/10.3324/haematol.2023.284325 PMid:38299578 PMCid:PMC11215383
- Martino
EA, Derudas D, Rossi E, Stefanoni P, Mangiacavalli S, Zamagni E,
Offidani M, Furlan A, Quinto AM, Della Pepa R, Bertuglia G, Barbieri E,
Conticello C, De Magistris C, Bongarzoni V, Cafro AM, Mele A, Botta C,
Sgherza N, Mele G, Annibali O, Rago A, Fontana R, Vigna E, Bruzzese A,
Mancuso K, Amendola A, Citro A, Cotzia E, More S, Rivolti E, Pettine L,
Galli M, De Stefano V, Petrucci MT, Corso A, Neri A, Di Raimondo F,
Bolli N, Musto P, Morabito F, Gentile M. Efficacy and Prognostic
Indicators of Isatuximab, Pomalidomide, and Dexamethasone (IsaPd) in
Daratumumab-Refractory Multiple Myeloma Patients: A Multicenter
Real-World Study. Hematol Oncol. 2025;43:e70042. https://doi.org/10.1002/hon.70042 PMid:39898517 PMCid:PMC11789454
- Richardson
PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, Lindsay J,
Weisel K, White D, Facon T, San Miguel J, Sunami K, O'Gorman P,
Sonneveld P, Robak P, Semochkin S, Schey S, Yu X, Doerr T, Bensmaine A,
Biyukov T, Peluso T, Zaki M, Anderson K, Dimopoulos M, investigators
Ot. Pomalidomide, bortezomib, and dexamethasone for patients with
relapsed or refractory multiple myeloma previously treated with
lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial.
Lancet Oncol. 2019;20:781-94. https://doi.org/10.1016/S1470-2045(19)30152-4 PMid:31097405
- Dimopoulos
M, Weisel K, Moreau P, Anderson LD, Jr., White D, San-Miguel J,
Sonneveld P, Engelhardt M, Jenner M, Corso A, Durig J, Pavic M, Salomo
M, Casal E, Srinivasan S, Yu X, Nguyen TV, Biyukov T, Peluso T,
Richardson P. Pomalidomide, bortezomib, and dexamethasone for multiple
myeloma previously treated with lenalidomide (OPTIMISMM): outcomes by
prior treatment at first relapse. Leukemia. 2021;35:1722-31. https://doi.org/10.1038/s41375-020-01021-3 PMid:32895455 PMCid:PMC8179841
- Richardson
P, Beksac M, Oriol A, Lindsay J, Schjesvold F, Galli M, Yagci M,
Larocca A, Weisel K, Yu X, Donahue C, Acosta J, Peluso T, Dimopoulos M.
Pomalidomide, Bortezomib, and Dexamethasone Versus Bortezomib and
Dexamethasone in Relapsed or Refractory Multiple Myeloma: Final
Survival and Subgroup Analyses From the OPTIMISMM Trial. Eur J
Haematol. 2025. https://doi.org/10.1111/ejh.14365 PMid:39777934
- Palumbo
A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I,
Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin
X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P, Investigators C.
Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl
J Med. 2016;375:754-66. https://doi.org/10.1056/NEJMoa1606038 PMid:27557302
- Mateos
MV, Sonneveld P, Hungria V, Nooka AK, Estell JA, Barreto W, Corradini
P, Min CK, Medvedova E, Weisel K, Chiu C, Schecter JM, Amin H, Qin X,
Ukropec J, Kobos R, Spencer A. Daratumumab, Bortezomib, and
Dexamethasone Versus Bortezomib and Dexamethasone in Patients With
Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR.
Clin Lymphoma Myeloma Leuk. 2020;20:509-18. https://doi.org/10.1016/j.clml.2019.09.623 PMid:32482541
- Dimopoulos
MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, Facon T, Ludwig H,
Oriol A, Goldschmidt H, Rosinol L, Straub J, Suvorov A, Araujo C,
Rimashevskaya E, Pika T, Gaidano G, Weisel K, Goranova-Marinova V,
Schwarer A, Minuk L, Masszi T, Karamanesht I, Offidani M, Hungria V,
Spencer A, Orlowski RZ, Gillenwater HH, Mohamed N, Feng S, Chng WJ,
Investigators E. Carfilzomib and dexamethasone versus bortezomib and
dexamethasone for patients with relapsed or refractory multiple myeloma
(ENDEAVOR): a randomised, phase 3, open-label, multicentre study.
Lancet Oncol. 2016;17:27-38. https://doi.org/10.1016/S1470-2045(15)00464-7 PMid:26671818
- Moreau
P, Joshua D, Chng WJ, Palumbo A, Goldschmidt H, Hajek R, Facon T,
Ludwig H, Pour L, Niesvizky R, Oriol A, Rosinol L, Suvorov A, Gaidano
G, Pika T, Weisel K, Goranova-Marinova V, Gillenwater HH, Mohamed N,
Aggarwal S, Feng S, Dimopoulos MA. Impact of prior treatment on
patients with relapsed multiple myeloma treated with carfilzomib and
dexamethasone vs bortezomib and dexamethasone in the phase 3 ENDEAVOR
study. Leukemia. 2017;31:115-22. https://doi.org/10.1038/leu.2016.186 PMid:27491641 PMCid:PMC5220137
- Sonneveld
P, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I,
Hungria V, Munder M, Mateos MV, Mark TM, Levin MD, Ahmadi T, Qin X,
Garvin Mayo W, Gai X, Carey J, Carson R, Spencer A. Overall Survival
With Daratumumab, Bortezomib, and Dexamethasone in Previously Treated
Multiple Myeloma (CASTOR): A Randomized, Open-Label, Phase III Trial. J
Clin Oncol. 2023;41:1600-9. https://doi.org/10.1200/JCO.21.02734 PMid:36413710 PMCid:PMC10022857
- Orlowski
RZ, Moreau P, Niesvizky R, Ludwig H, Oriol A, Chng WJ, Goldschmidt H,
Yang Z, Kimball AS, Dimopoulos M. Carfilzomib-Dexamethasone Versus
Bortezomib-Dexamethasone in Relapsed or Refractory Multiple Myeloma:
Updated Overall Survival, Safety, and Subgroups. Clin Lymphoma Myeloma
Leuk. 2019;19:522-30 e1. https://doi.org/10.1016/j.clml.2019.04.018 PMid:31160237
- Grosicki
S, Simonova M, Spicka I, Pour L, Kriachok I, Gavriatopoulou M,
Pylypenko H, Auner HW, Leleu X, Doronin V, Usenko G, Bahlis NJ, Hajek
R, Benjamin R, Dolai TK, Sinha DK, Venner CP, Garg M, Gironella M,
Jurczyszyn A, Robak P, Galli M, Wallington-Beddoe C, Radinoff A,
Salogub G, Stevens DA, Basu S, Liberati AM, Quach H, Goranova-Marinova
VS, Bila J, Katodritou E, Oliynyk H, Korenkova S, Kumar J, Jagannath S,
Moreau P, Levy M, White D, Gatt ME, Facon T, Mateos MV, Cavo M, Reece
D, Anderson LD, Jr., Saint-Martin JR, Jeha J, Joshi AA, Chai Y, Li L,
Peddagali V, Arazy M, Shah J, Shacham S, Kauffman MG, Dimopoulos MA,
Richardson PG, Delimpasi S. Once-per-week selinexor, bortezomib, and
dexamethasone versus twice-per-week bortezomib and dexamethasone in
patients with multiple myeloma (BOSTON): a randomised, open-label,
phase 3 trial. Lancet. 2020;396:1563-73. https://doi.org/10.1016/S0140-6736(20)32292-3 PMid:33189178
- Mateos
MV, Engelhardt M, Leleu X, Mesa MG, Cavo M, Dimopoulos M, Bianco M,
Merlo GM, Porte C, Richardson PG, Moreau P. Impact of prior treatment
on selinexor, bortezomib, dexamethasone outcomes in patients with
relapsed/refractory multiple myeloma: Extended follow-up subgroup
analysis of the BOSTON trial. Eur J Haematol. 2024;113:242-52. https://doi.org/10.1111/ejh.14223 PMid:38693052
- Liu
Y, Yang R, Feng H, Du Y, Yang B, Zhang M, He P, Ma B, Niu F. Adverse
events reporting of XPO1 inhibitor - selinexor: a real-word analysis
from FAERS database. Sci Rep. 2024;14:12231. https://doi.org/10.1038/s41598-024-62852-z PMid:38806549 PMCid:PMC11133441
- Lonial
S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, Abdallah AO,
Callander N, Lendvai N, Sborov D, Suvannasankha A, Weisel K, Karlin L,
Libby E, Arnulf B, Facon T, Hulin C, Kortum KM, Rodriguez-Otero P,
Usmani SZ, Hari P, Baz R, Quach H, Moreau P, Voorhees PM, Gupta I, Hoos
A, Zhi E, Baron J, Piontek T, Lewis E, Jewell RC, Dettman EJ, Popat R,
Esposti SD, Opalinska J, Richardson P, Cohen AD. Belantamab mafodotin
for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm,
randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207-21. https://doi.org/10.1016/S1470-2045(19)30788-0 PMid:31859245
- Lonial
S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, Abdallah AO,
Callander N, Sborov D, Suvannasankha A, Weisel K, Voorhees PM,
Womersley L, Baron J, Piontek T, Lewis E, Opalinska J, Gupta I, Cohen
AD. Longer term outcomes with single-agent belantamab mafodotin in
patients with relapsed or refractory multiple myeloma: 13-month
follow-up from the pivotal DREAMM-2 study. Cancer. 2021;127:4198-212. https://doi.org/10.1002/cncr.33809 PMid:34314018 PMCid:PMC8597112
- Hungria
V, Robak P, Hus M, Zherebtsova V, Ward C, Ho PJ, Ribas de Almeida AC,
Hajek R, Kim K, Grosicki S, Sia H, Bryant A, Pitombeira de Lacerda M,
Aparecida Martinez G, Sureda Balari AM, Sandhu I, Cerchione C, Ganly P,
Dimopoulos M, Fu C, Garg M, Abdallah AO, Oriol A, Gatt ME, Cavo M,
Rifkin R, Fujisaki T, Mielnik M, Pirooz N, McKeown A, McNamara S, Zhou
X, Nichols M, Lewis E, Rogers R, Baig H, Eccersley L, Roy-Ghanta S,
Opalinska J, Mateos MV, Investigators D-. Belantamab Mafodotin,
Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med.
2024;391:393-407. https://doi.org/10.1056/NEJMoa2405090 PMid:38828933
- Dimopoulos
MA, Beksac M, Pour L, Delimpasi S, Vorobyev V, Quach H, Spicka I,
Radocha J, Robak P, Kim K, Cavo M, Suzuki K, Morris K, Pompilus F,
Phillips-Jones A, Zhou XL, Fulci G, Sule N, Kremer BE, Opalinska J,
Mateos MV, Trudel S, Investigators D-. Belantamab Mafodotin,
Pomalidomide, and Dexamethasone in Multiple Myeloma. N Engl J Med.
2024;391:408-21. https://doi.org/10.1056/NEJMoa2403407 PMid:38828951
- Beksac
M. GGE, Delimpasi S., Robak P., Karunanithi K., De Arriba F., Radocha
J., Kim K., Voloshin S., Zherebtsova Z., Osipov O., Mateos M.V., Zafar
S.F., Pour L., Spicka I., Suzuki K., Du X., Wilkes J., Maroj B., Morris
K., Ma J., Polinkovsky M., Wang Z., Zhou X.L., Fulci G., Sule N.,
Kremer B., Opalinska J., Trudel S., Dimopoulos M.A. Belantamab
Mafodotin Plus Pomalidomide and Dexamethasone Vs Pomalidomide Plus
Bortezomib and Dexamethasone in Patients with Relapsed/Refractory
Multiple Myeloma: A Subset Analysis in Patients Who Have Received 1
Prior Line of Therapy Including Lenalidomide. Vol 144: Blood;
2024:4731. https://doi.org/10.1182/blood-2024-199282
- Dimopoulos
MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, Delforge M,
Hajek R, Schjesvold F, Cavo M, Goldschmidt H, Facon T, Einsele H,
Boccadoro M, San-Miguel J, Sonneveld P, Mey U. Multiple Myeloma:
EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and
Follow-up. Hemasphere. 2021;5:e528. https://doi.org/10.1016/j.annonc.2020.11.014 PMid:33549387
- Mikhael
J, Belhadj-Merzoug K, Hulin C, Vincent L, Moreau P, Gasparetto C, Pour
L, Spicka I, Vij R, Zonder J, Atanackovic D, Gabrail N, Martin TG,
Perrot A, Bensfia S, Weng Q, Brillac C, Semiond D, Mace S, Corzo KP,
Leleu X. A phase 2 study of isatuximab monotherapy in patients with
multiple myeloma who are refractory to daratumumab. Blood Cancer J.
2021;11:89. https://doi.org/10.1038/s41408-021-00478-4 PMid:33980831 PMCid:PMC8116334
- Dimopoulos
MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, Leleu X,
LeBlanc R, Suzuki K, Raab MS, Richardson PG, Popa McKiver M, Jou YM,
Shelat SG, Robbins M, Rafferty B, San-Miguel J. Elotuzumab plus
Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med.
2018;379:1811-22. https://doi.org/10.1056/NEJMoa1805762 PMid:30403938
- Dimopoulos
MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, Leleu X,
LeBlanc R, Suzuki K, Raab MS, Richardson PG, Popa McKiver M, Jou YM,
Yao D, Das P, San-Miguel J. Elotuzumab Plus Pomalidomide and
Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall
Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial. J Clin
Oncol. 2023;41:568-78. https://doi.org/10.1200/JCO.21.02815 PMid:35960908 PMCid:PMC9870233
- Gentile
M, Vigna E, Palmieri S, Galli M, Derudas D, Mina R, Della Pepa R,
Zambello R, Martino EA, Bruzzese A, Mangiacavalli S, Zamagni E,
Califano C, Musso M, Conticello C, Cerchione C, Mele G, Di Renzo N,
Offidani M, Tarantini G, Casaluci GM, Rago A, Ria R, Uccello G, Barila
G, Palumbo G, Pompa A, Vincelli D, Brunori M, Accardi F, Amico V,
Amendola A, Fontana R, Bongarzoni V, Rossini B, Cotzia E, Gozzetti A,
Rizzi R, Sgherza N, Ferretti E, Bertuglia G, Nappi D, Petrucci MT, Di
Raimondo F, Neri A, Morabito F, Musto P. Elotuzumab plus pomalidomide
and dexamethasone in relapsed/refractory multiple myeloma: a
multicenter, retrospective, real-world experience with 200 cases
outside of controlled clinical trials. Haematologica. 2024;109:245-55. https://doi.org/10.3324/haematol.2023.283251
- Martino
EA, Palmieri S, Galli M, Derudas D, Mina R, Della Pepa R, Zambello R,
Vigna E, Bruzzese A, Mangiacavalli S, Zamagni E, Califano C, Musso M,
Conticello C, Cerchione C, Mele G, Di Renzo N, Offidani M, Tarantini G,
Casaluci GM, Rago A, Ria R, Uccello G, Barila G, Palumbo G, Pettine L,
De Magistris C, Vincelli ID, Brunori M, Accardi F, Amico V, Amendola A,
Fontana R, Bongarzoni V, Rossini B, Cotzia E, Gozzetti A, Rizzi R,
Sgherza N, Curci P, Mancuso K, Reddiconto G, Maroccia A, Franceschini
L, Bertuglia G, Nappi D, Barbieri E, Quaresima M, Petrucci MT, Di
Raimondo F, Neri A, Tripepi G, Musto P, Morabito F, Gentile M. Outcomes
and prognostic indicators in daratumumab-refractory multiple myeloma: a
multicenter real-world study of elotuzumab, pomalidomide, and
dexamethasone in 247 patients. ESMO Open. 2025;10:104084. https://doi.org/10.1016/j.esmoop.2024.104084 PMid:39778329 PMCid:PMC11761902
- Schjesvold
FH, Dimopoulos MA, Delimpasi S, Robak P, Coriu D, Legiec W, Pour L,
Spicka I, Masszi T, Doronin V, Minarik J, Salogub G, Alekseeva Y,
Lazzaro A, Maisnar V, Mikala G, Rosinol L, Liberati AM, Symeonidis A,
Moody V, Thuresson M, Byrne C, Harmenberg J, Bakker NA, Hajek R, Mateos
MV, Richardson PG, Sonneveld P, Investigators O. Melflufen or
pomalidomide plus dexamethasone for patients with multiple myeloma
refractory to lenalidomide (OCEAN): a randomised, head-to-head,
open-label, phase 3 study. Lancet Haematol. 2022;9:e98-e110.
- Schjesvold
FH, Ludwig H, Mateos MV, Larocca A, Abdulhaq H, Norin S, Thuresson M,
Bakker NA, Richardson PG, Sonneveld P. Melflufen in relapsed/refractory
multiple myeloma refractory to prior alkylators: A subgroup analysis
from the OCEAN study. Eur J Haematol. 2024;112:402-11. https://doi.org/10.1111/ejh.14127 PMid:37968873
- Munshi
NC, Anderson LD, Jr., Shah N, Madduri D, Berdeja J, Lonial S, Raje N,
Lin Y, Siegel D, Oriol A, Moreau P, Yakoub-Agha I, Delforge M, Cavo M,
Einsele H, Goldschmidt H, Weisel K, Rambaldi A, Reece D, Petrocca F,
Massaro M, Connarn JN, Kaiser S, Patel P, Huang L, Campbell TB, Hege K,
San-Miguel J. Idecabtagene Vicleucel in Relapsed and Refractory
Multiple Myeloma. N Engl J Med. 2021;384:705-16. https://doi.org/10.1056/NEJMoa2024850 PMid:33626253
- Dima
D, Rashid A, Davis JA, Shune L, Abdallah AO, Li H, DeJarnette S, Khouri
J, Williams L, Hashmi H, Raza S, McGuirk J, Anwer F, Ahmed N. Efficacy
and safety of idecabtagene vicleucel in patients with
relapsed-refractory multiple myeloma not meeting the KarMMa-1 trial
eligibility criteria: A real-world multicentre study. Br J Haematol.
2024;204:1293-9. https://doi.org/10.1111/bjh.19302 PMid:38263627
- Rodriguez-Otero
P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S,
Callander N, Costa LJ, Vij R, Bahlis NJ, Moreau P, Solomon SR, Delforge
M, Berdeja J, Truppel-Hartmann A, Yang Z, Favre-Kontula L, Wu F,
Piasecki J, Cook M, Giralt S. Ide-cel or Standard Regimens in Relapsed
and Refractory Multiple Myeloma. N Engl J Med. 2023;388:1002-14. https://doi.org/10.1056/NEJMoa2213614 PMid:36762851
- Ailawadhi
S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S, Callander N, Costa
LJ, Vij R, Bahlis NJ, Moreau P, Solomon S, Abrahamsen IW, Baz R, Broijl
A, Chen C, Jagannath S, Raje N, Scheid C, Delforge M, Benjamin R, Pabst
T, Iida S, Berdeja J, Giralt S, Truppel-Hartmann A, Chen Y, Zhong X, Wu
F, Piasecki J, Eliason L, Dhanda D, Felten J, Caia A, Cook M, Popa
McKiver M, Rodriguez-Otero P. Ide-cel vs standard regimens in
triple-class-exposed relapsed and refractory multiple myeloma: updated
KarMMa-3 analyses. Blood. 2024;144:2389-401. https://doi.org/10.1182/blood.2024024582 PMid:39197072
- Usmani
S. PK, Hari P., Berdeja J., Alsina M., Vij R., Raje N., Leleu X,
Dhodapkar M., Reshef R., Truppel-Hartmann A., Basudhar D., Thompson E.,
Zheng X., Ananthakrishnan R., Greggio C., Favre-Kontula L., Sternas L.,
San-Miguel J. KarMMa-2 Cohort 2a: Efficacy and Safety of Idecabtagene
Vicleucel in Clinical High-Risk Multiple Myeloma Patients with Early
Relapse after Frontline Autologous Stem Cell Transplantation. Vol 140:
Blood; 2022:875-7. https://doi.org/10.1182/blood-2022-162469
- Dhodapkar
M. AM, Berdeja J., Patel K., Vij R., Leleu X., Truppel-Hartmann A.,
Basudhar D., Thompson E., Zheng X., Ananthakrishnan R., Favre-Kontula
L., Greggio C., Sternas L., Siegel D. KarMMa-2 Cohort 2c: Efficacy and
Safety of Idecabtagene Vicleucel in Patients with Clinical High-Risk
Multiple Myeloma Due to Inadequate Response to Frontline Autologous
Stem Cell Transplantation. Vol 140: Blood; 2022:7441-3. https://doi.org/10.1182/blood-2022-162615
- Berdeja
JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK,
Hari P, Htut M, Lesokhin A, Deol A, Munshi NC, O'Donnell E, Avigan D,
Singh I, Zudaire E, Yeh TM, Allred AJ, Olyslager Y, Banerjee A, Jackson
CC, Goldberg JD, Schecter JM, Deraedt W, Zhuang SH, Infante J, Geng D,
Wu X, Carrasco-Alfonso MJ, Akram M, Hossain F, Rizvi S, Fan F, Lin Y,
Martin T, Jagannath S. Ciltacabtagene autoleucel, a B-cell maturation
antigen-directed chimeric antigen receptor T-cell therapy in patients
with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase
1b/2 open-label study. Lancet. 2021;398:314-24. https://doi.org/10.1016/S0140-6736(21)00933-8 PMid:34175021
- Martin
T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, Avigan D, Deol A,
Htut M, Lesokhin A, Munshi NC, O'Donnell E, Stewart AK, Schecter JM,
Goldberg JD, Jackson CC, Yeh TM, Banerjee A, Allred A, Zudaire E,
Deraedt W, Olyslager Y, Zhou C, Pacaud L, Madduri D, Jakubowiak A, Lin
Y, Jagannath S. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation
Antigen Chimeric Antigen Receptor T-Cell Therapy, for
Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J
Clin Oncol. 2023;41:1265-74. https://doi.org/10.1200/JCO.22.00842 PMid:35658469 PMCid:PMC9937098
- San-Miguel
J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, Fernandez de
Larrea C, Martinez-Lopez J, Moreau P, Touzeau C, Leleu X, Avivi I, Cavo
M, Ishida T, Kim SJ, Roeloffzen W, van de Donk N, Dytfeld D, Sidana S,
Costa LJ, Oriol A, Popat R, Khan AM, Cohen YC, Ho PJ, Griffin J,
Lendvai N, Lonardi C, Slaughter A, Schecter JM, Jackson CC, Connors K,
Li K, Zudaire E, Chen D, Gilbert J, Yeh TM, Nagle S, Florendo E, Pacaud
L, Patel N, Harrison SJ, Einsele H. Cilta-cel or Standard Care in
Lenalidomide-Refractory Multiple Myeloma. N Engl J Med.
2023;389:335-47. https://doi.org/10.1056/NEJMoa2303379 PMid:37272512
- Mateos
M-V, San-Miguel J, Dhakal B, Touzeau C, Leleu X, van de Donk NWCJ,
Sidana S, Oriol A, Cohen YC, Harrison SJ, Einsele H, Corradini P, Chen
D, Li Q, Li K, Slaughter A, Lonardi C, Benachour N, Vogel M, Lendvai N,
Koneru M, Patel N, Florendo E, Ho PJ, Popat R. OA-65 Overall Survival
(OS) With Ciltacabtagene Autoleucel (Cilta-cel) Versus Standard of Care
(SoC) in Lenalidomide (Len)-Refractory Multiple Myeloma (MM): Phase 3
CARTITUDE-4 Study Update. Clinical Lymphoma, Myeloma and Leukemia.
2024;24:S290. https://doi.org/10.1016/S2152-2650(24)02346-2
- Moreau
P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, Nooka AK,
Martin T, Rosinol L, Chari A, Karlin L, Benboubker L, Mateos MV, Bahlis
N, Popat R, Besemer B, Martinez-Lopez J, Sidana S, Delforge M, Pei L,
Trancucci D, Verona R, Girgis S, Lin SXW, Olyslager Y, Jaffe M, Uhlar
C, Stephenson T, Van Rampelbergh R, Banerjee A, Goldberg JD, Kobos R,
Krishnan A, Usmani SZ. Teclistamab in Relapsed or Refractory Multiple
Myeloma. N Engl J Med. 2022;387:495-505. https://doi.org/10.1056/NEJMoa2203478 PMid:35661166 PMCid:PMC10587778
- Varma
G, Fogel L, Gordon B, Saldarriaga MM, Ahn J, Aleman A, Caro J,
Rosenberg MC, Monge J, Parmar H, Kaminetzky D, Moskovits T, Siegel DS,
Morgan GJ, Niesvizky R, Davies FE, Biran N. Real-world safety and
efficacy of teclistamab in relapsed/refractory multiple myeloma:
results from a multicenter, retrospective study and descriptive
meta-analysis. Leuk Lymphoma. 2025:1-10. https://doi.org/10.1080/10428194.2024.2446617 PMid:39756041
- Lesokhin
AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R,
Rodriotaguez-Otero P, Martinez-Lopez J, Koehne G, Touzeau C, Jethava Y,
Quach H, Depaus J, Yokoyama H, Gabayan AE, Stevens DA, Nooka AK, Manier
S, Raje N, Iida S, Raab MS, Searle E, Leip E, Sullivan ST, Conte U,
Elmeliegy M, Czibere A, Viqueira A, Mohty M. Elranatamab in relapsed or
refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat
Med. 2023;29:2259-67. https://doi.org/10.1038/s41591-023-02528-9 PMid:37582952 PMCid:PMC10504075
- Tomasson
MH, Iida S, Niesvizky R, Mohty M, Bahlis NJ, Martinez-Lopez J, Koehne
G, Rodriguez-Otero P, Miles Prince H, Viqueira A, Leip E, Conte U,
Sullivan ST, Lesokhin AM. Long-term survival and safety of elranatamab
in patients with relapsed or refractory multiple myeloma: Update from
the MagnetisMM-3 study. Hemasphere. 2024;8:e136. https://doi.org/10.1002/hem3.136 PMid:39055646 PMCid:PMC11269363
- Chari
A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodriguez-Otero P,
Askari E, Mateos MV, Costa LJ, Caers J, Verona R, Girgis S, Yang S,
Goldsmith RB, Yao X, Pillarisetti K, Hilder BW, Russell J, Goldberg JD,
Krishnan A. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific
Antibody for Multiple Myeloma. N Engl J Med. 2022;387:2232-44. https://doi.org/10.1056/NEJMoa2204591 PMid:36507686
- Ferreri
CJ, Hildebrandt MAT, Hashmi H, Shune LO, McGuirk JP, Sborov DW, Wagner
CB, Kocoglu MH, Rapoport A, Atrash S, Voorhees PM, Khouri J, Dima D,
Afrough A, Kaur G, Anderson LD, Jr., Simmons G, Davis JA, Kalariya N,
Peres LC, Lin Y, Janakiram M, Nadeem O, Alsina M, Locke FL, Sidana S,
Hansen DK, Patel KK, Castaneda Puglianini OA. Real-world experience of
patients with multiple myeloma receiving ide-cel after a prior
BCMA-targeted therapy. Blood Cancer J. 2023;13:117. https://doi.org/10.1038/s41408-023-00886-8 PMid:37558706 PMCid:PMC10412575
- Dhakal
B AOS, Cowan A.J., Richard S., Friend R., Rees M.J., Costello P.,
Martinez M.V., Pasvolsky O., Wagner C.O., Jensen A., Davis J.A., Reshef
R., Dima D., Banerjee R., Bhutani M., Nadeem O., Parrondo R.D.,
Mikkilineni L., Raza S., Kapoor P., Hosoya H., Chhabra S.,
Grajales-Cruz A., Gaballa M.R., Midha S. Alsina M, Sborov D.W., Patel
K.K., Lin Y., Ferreri C.J., Hansen D.K., Costa L.J., Sidana S.
Talquetamab Bridging: Paving the Way to B-Cell Maturation Antigen
(BCMA) CAR-T Cell Therapy in Relapsed/Refractory Multiple Myeloma
(RRMM). 66th ASH Annual Meeting Blood; 2024. p. 931. https://doi.org/10.1182/blood-2024-202017
- Lonial
S, Popat R, Hulin C, Jagannath S, Oriol A, Richardson PG, Facon T,
Weisel K, Larsen JT, Minnema MC, Abdallah AO, Badros AZ, Knop S,
Stadtmauer EA, Cheng Y, Amatangelo M, Chen M, Nguyen TV, Amin A, Peluso
T, van de Donk N. Iberdomide plus dexamethasone in heavily pretreated
late-line relapsed or refractory multiple myeloma (CC-220-MM-001): a
multicentre, multicohort, open-label, phase 1/2 trial. Lancet Haematol.
2022;9:e822-e32. https://doi.org/10.1016/S2352-3026(22)00290-3 PMid:36209764
- Richardson
PG, Trudel S, Popat R, Mateos MV, Vangsted AJ, Ramasamy K,
Martinez-Lopez J, Quach H, Orlowski RZ, Arnao M, Lonial S, Karanes C,
Pawlyn C, Kim K, Oriol A, Berdeja JG, Rodriguez Otero P, Casas-Aviles
I, Spirli A, Poon J, Li S, Gong J, Wong L, Lamba M, Pierce DW,
Amatangelo M, Peluso T, Maciag P, Katz J, Pourdehnad M, Bahlis NJ,
Investigators C-M-S. Mezigdomide plus Dexamethasone in Relapsed and
Refractory Multiple Myeloma. N Engl J Med. 2023;389:1009-22. https://doi.org/10.1056/NEJMoa2303194 PMid:37646702
- Mele
G, Sgherza N, Pastore D, Musto P. Strengths and Weaknesses of Different
Therapeutic Strategies for the Treatment of Patients with Multiple
Myeloma Who Progress After the Frontline Use of Lenalidomide: A
Narrative Review. J Clin Med. 2024;13. https://doi.org/10.3390/jcm13206238 PMid:39458188 PMCid:PMC11508845