The immune system, as mentioned, carries out its functions through two main modalities: humoral and cellular mediators: innate immunity and acquired immunity.[1]
During infection, innate immunity is responsible for an immediate, nonspecific response against a wide range of pathogens and is considered the first line of defense in non-immunized individuals. This type of defense mechanism is common to all multicellular organisms, including insects and plants.
Adaptive (specific) immunity develops as a result of the immune system's contact with microorganisms or external substances (antigens). Later, components of acquired immunity will develop a memory for specific antigens, producing antibodies against them. After the first exposure to a new antigen, the development of acquired immunity takes time. Once this mechanism is established, the specific antigen is remembered, and subsequent responses to it are faster and more effective than the first exposure. The main effectors of this immune response are T lymphocytes and B lymphocytes, which produce antibodies, as well as dendritic cells, cytokines, and the complement system.
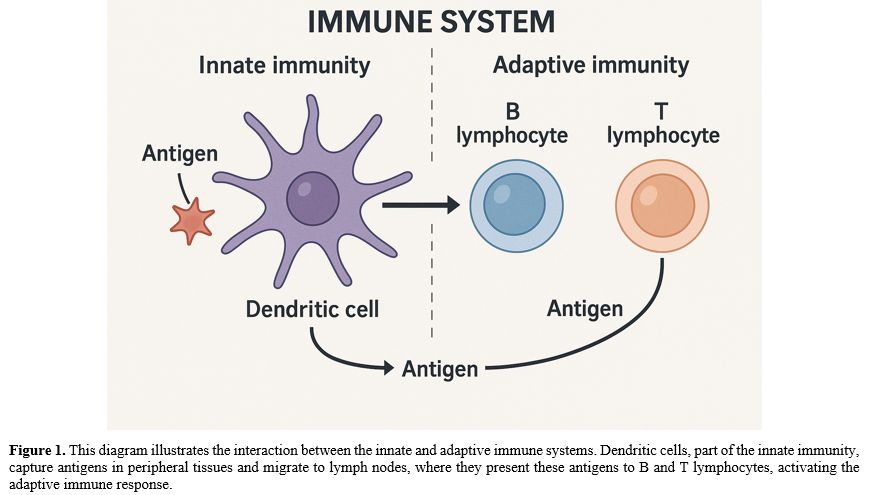
These two systems, innate and acquired, are strongly interconnected and cooperate to ensure the immune system functions properly; for example, some immune cells, such as dendritic cells, are responsible for binding surface antigens in tissues and presenting them to B lymphocytes, or for internalizing and processing them before presenting them to T lymphocytes (Figure 1).
An immunological response to an infection is always associated with an inflammatory reaction. Similarly, both acute and chronic inflammatory processes are always accompanied by immune system involvement.[2]
Therefore, a functioning immune system is essential to counteract infections. Understanding the clinical picture of patients with infectious diseases is crucial. A weakened defense system could favor infections, as is the case with elderly patients, those with diabetes, or those with acquired immunological deficits. On the other hand, many individuals with abnormal immunologic responses to biological agents experience lethal outcomes or damage due to overly intense or misdirected immune reactions.
A more recent example can be observed during the COVID-19 pandemic. Recurring lethal complications in COVID-19 were characterized by hyperactivation of the immune system, sustained by a massive release of proinflammatory cytokines, which resulted in multiorgan failure.[3]
During acute and/or chronic infections, immune function can be suppressed, slowing healing and promoting recurrence and/or coinfections.[4] Individuals with poorly functioning immune systems, such as primary and secondary immunodeficiency, are more susceptible to infection and autoimmunity.[5] Consequently, increasing the efficiency of the immune system is a strategy to consider in treating infections.
An "efficient" immune system is essential to prevent infections and avoid uncontrolled responses, particularly in individuals with suboptimal immune responses. In this scenario, scientific and pharmacological research plays a fundamental role in developing innovative therapeutic strategies that promote optimal immune responses.
Over the past 20 years, many products have been proposed as "immunostimulants," "immunomodulators," or "adjuvants”. These products are substances that can provide adequate immunity against harmful antigens and enhance the body's response to infections. These modifiers of immune reactivity are divided into natural physiological substances e.g., interferons, thymic factors, lactoferrin, probiotics), exogenous substances (e.g., bacterial lysates, milk enzymes), and synthetic substances (e.g., liposomes, imiquimod, pidotimod). These substances act differently on the immune system and produce varying results, so they should not be considered equivalent.
Synthetic immunomodulators can stimulate a more rapid and effective immune response. They interact directly with T lymphocyte receptors, leading to increased expression and activity. Among these, Pidotimod is a dipeptide consisting of L-pyroglutamic acid and L-thiazolidin-4-carboxylic acid. It can induce dendritic cell maturation, increase phagocytic activity and chemotaxis of macrophages and neutrophils (natural immunity), increase the number of T lymphocytes (cell-mediated immunity), stimulate differentiation toward a Th1 phenotype, activate NK lymphocytes, and stimulate B lymphocytes to increase antibody production.
Pidotimod has an oral bioavailability of 44% and a plasma clearance of 5 L/h. Its plasma half-life is about 4 hours, and it takes 1.3-2 hours to reach maximum plasma concentration. Oral bioavailability is reduced by up to 50% when co-administered with a meal, compared with its administration in a fasting state. It undergoes minimal hepatic metabolism, and the administered dose is excreted by the kidneys unchanged (95%). Pidotimod has shown low plasma protein binding and is not significantly metabolized, so no significant pharmacokinetic interactions are expected.[6,7] It has very low toxicity, with animal studies showing that it is not mutagenic or teratogenic in rats and rabbits, does not affect fertility, and has no peri- or postnatal toxicity in rats. While often used interchangeably, an "immunostimulant" broadly enhances immune responses, whereas an "immunomodulator" fine-tunes or balances them. Pidotimod is typically classified as an immunomodulator due to its ability to both stimulate and regulate various aspects of the immune system.
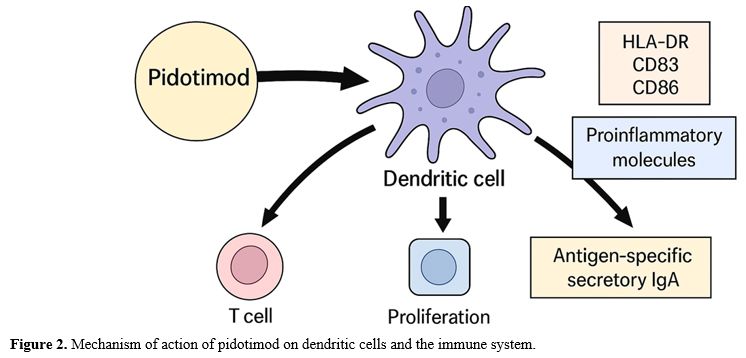
Several studies show that Pidotimod can influence innate and adaptive immunity, modulating the immune response in different clinical conditions, as depicted in Figure 2. Early human studies on innate immunity have shown that Pidotimod induces bactericidal activity in alveolar macrophages and activates innate immunity at the level of natural killer cell activity.[8,9] It also can affect dendritic cell (DC) maturation and increase the expression of the major histocompatibility complex class II cell surface receptor (HLA-DR), costimulatory molecules CD83 and CD86, and the production of proinflammatory molecules such as monocyte chemoattractant protein-1 and tumor necrosis factor (TNF)-α, which drive T cell proliferation and differentiation toward a Th1 phenotype.[10] The primary trigger for Pidotimod is the stimulation of Toll-like receptors (TLRs), which activate an innate immune response. Regarding adaptive immunity, Pidotimod increases the production of antigen-specific secretory immunoglobulin A (IgA) (Figure 2).[11]
Recent research has shown that Pidotimod can activate the G protein-coupled chemokine receptor CXC3, binding to guanosine-triphosphate-binding proteins and coordinating the T cell response in inflamed tissues, with a multifaceted mechanism of action.[12]
Other studies have shown that Pidotimod enhanced the anti-growth effect of cisplatin in a Lewis lung cancer model by promoting an antitumor response, with increased infiltration of DC and CD8+ T cells as well as enhanced expression of interferon (IFN)-ƴ.[13]
Recent reviews on Pidotimod have stated that it shows an optimal safety profile, with no increased frequency of reported adverse reactions or autoimmune disorders in treated patients. A recent review confirmed that Pidotimod has a good local and systemic tolerability in children; vomiting, diarrhea, abdominal pain, erythema, and lack of appetite are the majority of adverse events reported as transient, with no significant difference between Pidotimod and placebo in the incidence of drug-related adverse events, indicating that long-term use of this immunostimulatory drug is safe.[14]
Pidotimod in Children: Key Findings
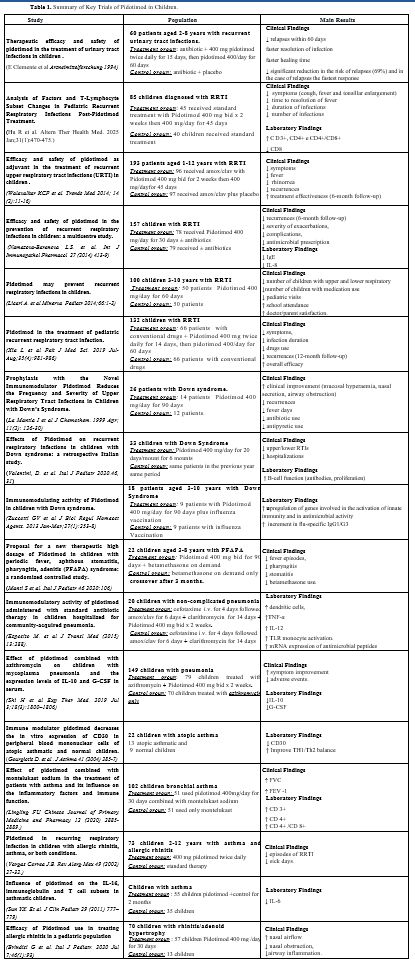
In the last decades, several studies have been conducted on the activity of Pidotimod in this population, considering various pathological conditions[15] as shown in a recent review (Table 1).[16]• Recurrent urinary tract infections. A multicenter, randomized, double-blind trial evaluated 60 patients (ages 2–8 years) with recurrent urinary tract infections. One group received a 15-day administration of antibiotics and Pidotimod 400 mg twice daily, followed by another 60 days of Pidotimod 400 mg once daily, while the other group received a placebo. In the 60-day follow-up, four relapses were observed in the Pidotimod-treated group and 13 in the placebo group. The infection resolution was faster in the Pidotimod group (6.9 days vs. 8.3 days), with a significantly reduced risk of relapse (69%) and a faster clinical response in these patients.[17]
• Recurrent Respiratory Tract Infections. Recurrent respiratory tract infections in children lead to increased absences from school, worse quality of life, and further respiratory complications, including bronchiectasis. For this reason, adequate prevention and treatment are essential. A recent model analysis showed that pidotimod is a cost-effective strategy to reduce the incidence rate of recurrent respiratory tract infections in children.[18] Many studies have documented the role of Pidotimod: in a retrospective cohort study of children with recurrent respiratory tract infections, symptoms such as cough, fever, wet rales, and enlarged tonsils resolved significantly faster with Pidotimod, with a reduction in recurrent infections. Moreover, post-treatment levels of CD3+, CD4+, and CD4+/CD8+ were elevated in the Pidotimod group compared with the control group.[19] Another study in 100 children emphasized that Pidotimod could reduce the frequency and severity of infections, making it a valuable adjunct in paediatric prevention strategies.[20]
In a multicenter, prospective, randomized, double-blind controlled trial with 193 pediatric patients aged 1–12 years (mean age of 6.7) with recurrent respiratory infections and impaired immunologic function, a group treated with Pidotimod 400 mg x 2/day for 15 days plus Amoxicillin/Clavulanic acid followed by maintenance with Pidotimod 400 mg showed statistically significant improvements (p<0.05) in fever, ear pain, cough, expectoration, rhinorrhea, and otalgia scores compared to the placebo+antibiotic group after 30 days of maintenance. In conclusion, the study showed that the drug enables symptom relief in the acute phase compared with the placebo group and prevents episodes during the subsequent 30 days of maintenance.[21]
A randomized clinical trial conducted in Russia with children more susceptible to respiratory infections demonstrated that a 30-day course of Pidotimod led to a reduction in the number of acute respiratory infection episodes over a 6-month follow-up. The treatment group also exhibited faster recovery, with rapid symptom resolution and a normalization of serum immunological markers. Most notably, interleukin-8, a proinflammatory cytokine, normalized faster in the treatment group, confirming Pidotimod's immunomodulatory effect.[22] This data was also confirmed in a Pakistani clinical study, which showed that Pidotimod significantly decreased the number of infection episodes and improved immune parameters, such as immunoglobulin levels and T-cell subsets.[23]
A 2019 meta-analysis involving 29 RCTs and 4344 pediatric patients found that Pidotimod significantly reduced the frequency of recurrent respiratory infections. Specifically, the rate ratio (RR) for fewer infections was 1.59 (95% CI 1.45-1.74, p < 0.00001). It also lowered the use of antibiotics, improved serum immunoglobulin levels (IgG, IgA, IgM), and enhanced T lymphocyte subtypes (CD3+, CD4+). Furthermore, Pidotimod was not associated with an increased risk of adverse events (RR = 1.05, 95% CI 0.72-1.54, p = 0.80).[24]
• Down’s Syndrome. Several studies have investigated the immunomodulatory effects of Pidotimod in children with Down syndrome, a population particularly vulnerable to recurrent respiratory infections. La Mantia demonstrated that Pidotimod significantly reduced the frequency and severity of respiratory tract infections in this group, likely due to enhanced immune response.[25] Similarly, another study reported improvements in mucosal immunity and infection control, supporting the use of Pidotimod as a preventive therapy.[26] Additional evidence confirmed its role in modulating immune parameters, including T-cell function, suggesting that Pidotimod may contribute to strengthening both innate and adaptive immunity in children with Down syndrome.[27]
• Periodic fever, ulcerous stomatitis, pharyngitis, adenitis syndrome (PFAPA). A crossover study with 22 children suffering from PFAPA syndrome (a rare condition with few treatment options) showed that Pidotimod significantly reduced the frequency of fevers, episodes of pharyngitis, and aphthous stomatitis. The need for betamethasone (a corticosteroid) also decreased. 86.4% of patients showed improvement. No serious side effects were observed during the study period.[28]
• Community-acquired pneumonia. In a study of children hospitalized with community-acquired pneumonia, 20 children were randomized to receive Pidotimod, administered alongside standard antibiotics (cefotaxime + clarithromycin) or standard antibiotics alone. Blood samples were analyzed under the following conditions: without any stimulus, in the presence of a mixture of eight pneumococcal polysaccharides, or of lipopolysaccharide. Blood analysis revealed that Pidotimod improved the activation and costimulatory molecule expression in dendritic cells, resulting in higher secretion of TNF-α, IL-12, and enhanced TLR-2 expression, suggesting that Pidotimod enhances the immune response, potentially aiding in faster recovery and better handling of bacterial infections like pneumonia.[29] Another retrospective cohort study involving 149 children with Mycoplasma pneumonia comparing azithromycin alone versus azithromycin plus pidotimod, found that the combination therapy significantly increased overall treatment efficacy (94.9% vs. 81.4%, p < 0.05), shortened symptom resolution times, reduced adverse reactions, and lowered serum levels of inflammatory markers IL‑10 and G‑CSF more than azithromycin alone.[30]
• Asthma. Several studies have shown that Pidotimod helps improve the inflammatory profile in asthmatic children by reducing markers like IL-4, CD30, and IL-6, while increasing IgA levels. This results in a reduction in respiratory infections in asthmatic children.[31-36] One Chinese observational study showed that combining Pidotimod with Montelukast (a medication used for asthma) improved cough scores and FEV1 (a measure of lung function) in children, compared to the group treated with Montelukast alone. This combination therapy could offer synergistic benefits for children with asthma who suffer from frequent infections.[32] Interestingly, in a study of children aged 6 to 12 years with allergic rhinitis, Pidotimod significantly improved nasal airflow, bringing it to levels comparable to those of healthy controls in children with allergic rhinitis (AR). This clinical benefit occurred despite the absence of detectable alterations in the nasal microbiota, suggesting that the effect of pidotimod likely arises from modulation of local inflammatory processes rather than microbial alterations.[37]
Pidotimod in Adults: Key Findings
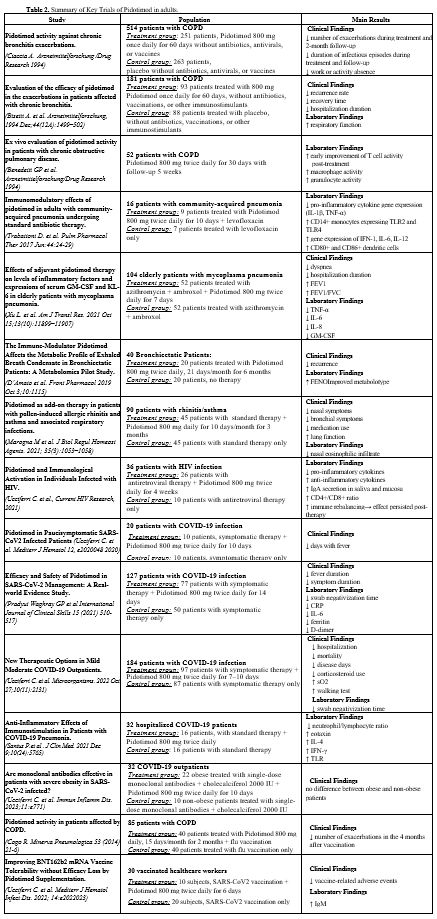
Few well-conducted studies in the adult patient guide the use of pidotimod in various pathological conditions (Table 2).[38,39]• Recurrent urinary tract infections. A multicenter, randomized, double-blin
• Chronic bronchitis. Represents the first disease in which the use of pidotimod in adults has been studied. Ciaccia et al. studied Pidotimod in adults with chronic bronchitis; subjects were randomized to receive pidotimod (n=251) or placebo (n=263) for 2 months, followed by 3 months of follow-up. Pidotimod-treated patients had longer intervals between exacerbations (105 vs. 98 days, P < 0.01), with reduced antibiotic use and fewer sick leaves.[40] These data were also confirmed in a multicenter, randomized, double-blind study involving 181 patients with chronic bronchitis, where the use of pidotimod significantly reduced the incidence of infectious exacerbation and hospitalizations.[41]
• Chronic Obstructive Pulmonary Disease (COPD). In a randomized, double-blind study enrolling 52 patients, Pidotimod (800 mg twice daily for 30 days) was shown to enhance T-cell activity in COPD patients. The effect on T-cell function was observed after 15 days of treatment and persisted for up to 5 weeks.[42]
• Pneumonia and Respiratory system. Trabattoni conducted a randomized controlled trial in hospitalized adults with community-acquired pneumonia (PSI III–IV or CURB‑65 0–2), adding pidotimod (800 mg twice daily) to standard levofloxacin therapy. Immunological assessments showed a significant up-regulation of antimicrobial/immunomodulatory proteins, increased percentages of cells expressing TLR-2, TLR-4, CD80, and CD86, and a marked reduction in TNF-α-producing cells.[43] Xu evaluated elderly patients with Mycoplasma pneumoniae infection. Adjunctive pidotimod led to a significant decline in inflammatory cytokines (including IL‑10 and G‑CSF), improved respiratory function, clinical recovery timelines, and lower reinfection rates compared to controls.[44] D'Amato explored metabolic changes in bronchiectasis patients receiving pidotimod. They demonstrated pidotimod altered metabolic pathways linked to inflammation and oxidative stress, suggesting it may mitigate exacerbation in chronic inflammatory lung disease.[45] Another study examined patients with allergic rhinitis and asthma and recurrent respiratory infections treated with pidotimod, highlighting a reduction in the frequency of infections, the use of antibiotics and corticosteroids, and improved immune parameters.[46]
• Human Immunodeficiency Syndrome (HIV). Antiretroviral therapy has significantly prolonged the lifetime of HIV-infected patients. However, individuals with HIV infection have residual systemic inflammation and persistent immune activation, with complications not pertaining to the classic manifestations of AIDS. A randomized trial with 40 HIV patients on antiretroviral therapy revealed that Pidotimod (800 mg twice daily for 4 weeks) led to a reduction in proinflammatory cytokines, with a corresponding increase in anti-inflammatory cytokines. This rebalancing also improved the CD4/CD8 ratio, salivary IgA secretion, and cystatin C levels, a marker for inflammation and cardiovascular risk. The immunologic benefits of Pidotimod persisted even after treatment cessation.[47]
• SARS CoV2 INFECTION/COVID-19. Several studies were produced during the Coronavirus disease 2019 (COVID-19) pandemic that significantly impacted human lives, and with which we, unfortunately, had to cope. During the first phase of the pandemic, there was considerable confusion among physicians and the public about how to manage this serious disease. Many treatments were proposed, but most proved ineffective. Early in the pandemic, several researchers explored Pidotimod as a potential therapy, given its immunomodulatory effects and potential to influence T lymphocyte responses, which are critical in fighting viral infections like SARS-CoV-2.[48] A predictive mathematical model also suggested that Pidotimod could play a role in eradicating the virus by modulating T lymphocyte responses to viral.[49] In the first half of 2020, initial clinical work was produced evaluating the role of Pidotimod in patients with COVID-19 who did not exhibit respiratory failure. The first clinical paper in early 2020 recruited 20 patients, who were randomized into two groups: one received symptomatic therapy plus Pidotimod (800 mg twice daily for 10 days), while the other group received symptomatic treatment only. The results were promising, with Pidotimod-treated patients showing faster clinical recovery, as evidenced by a significant reduction in fever duration (4.10 ± 2.18 days in the treatment group vs. 7.50 ± 2.63 days in the control group, P = 0.006).[50] Later, an Indian study with 140 patients (all without oxygen desaturation) confirmed these findings. The study reported that Pidotimod significantly reduced inflammatory markers and other biological indicators associated with COVID-19. The authors concluded that Pidotimod may play a significant role in managing SARS-CoV-2 infections, particularly in reducing the inflammatory response.[51]
A large retrospective case-control study involving 1231 unvaccinated patients with mild to moderate COVID-19 infection was conducted. From this cohort, 184 patients were selected and divided into two groups: 97 patients received Pidotimod (800 mg twice daily for 7-10 days) and 87 received only symptomatic therapy. Primary endpoints of the study included emergency room access for COVID-19-related pathology, hospitalizations, and deaths. The secondary outcome was the duration of illness. The results showed a 50% reduction in hospitalizations (P = 0.008) for patients treated with Pidotimod, shortened duration of illness (P = 0.005) in the treatment group, reduced corticosteroid use (P < 0.001), and improved oxygen saturation during the Walking Test (P = 0.01). Therefore, the authors concluded that Pidotimod can prevent worsening of COVID-19 infection and facilitate more rapid virologic clearance.[52]
In another study, Santus et al. examined the impact of Pidotimod on immunologic biomarkers and clinical severity in patients with mild to moderate COVID-19 pneumonia. Patients received standard care plus Pidotimod (800 mg twice daily). Results showed no significant differences in duration of hospitalization, mortality, or intubation rates compared to historical controls. Pidotimod-treated patients had a lower neutrophil-to-lymphocyte ratio (P = 0.037) and progressive increases in eotaxin and IL-4 levels (P < 0.05). In vitro, Pidotimod enhanced the expression of IFN-γ (P < 0.05) and TLR. The study suggested that Pidotimod may help accelerate healing by boosting the innate immune response to the viral infection.[53]
Further research evaluated the effects of co-administration of Pidotimod with monoclonal antibodies in obese COVID-19 patients (BMI ≥ 35 kg/m²) versus normal-weight patients. The study found that Pidotimod showed similar effects in both groups, despite obesity being an independent risk factor for hospitalization, suggesting that Pidotimod may provide benefits in both obese and non-obese individuals, regardless of BMI.[54]
• Vaccinations. In real practice, an interesting use of Pidotimod has shown promise as an adjuvant to vaccination. An excellent response to a vaccine is closely linked to a fully functioning immune system; however, vaccinations are used in individuals who often respond poorly to vaccines (elderly, immunocompromised patients, and children). Consequently, enhancing the immune response with immunomodulators could be a valuable strategy to improve vaccine efficacy.
In patients with COPD, it helped improve the immune response to influenza vaccination, leading to fewer flare-ups.[55] Similarly, in healthy adults receiving the COVID-19 vaccine, Pidotimod improved antibody responses and reduced vaccine side effects, particularly enhancing IgM production (P = 0.02), suggesting that Pidotimod could enhance immunological memory, potentially improving vaccine efficacy in populations prone to poor responses (e.g., elderly, immunocompromised).[56]
Conclusions
Respiratory Infections and Immunomodulatory Effects. Most acute respiratory tract infections are viral, and in this circumstance, the widespread misuse of antibiotics has contributed to the rise of antibiotic resistance. To counter this, there is increasing interest in alternative approaches such as enhancing the immune response, especially in vulnerable populations like the young and the elderly. Pidotimod, an immunostimulant, has shown potential in this context.A 2019 review highlighted Pidotimod's immunostimulatory properties and its pharmacokinetic profile, emphasizing its role in treating and preventing acute respiratory infections. The authors conclude that Pidotimod was effective in:
• Reducing reinfection rates (OR 0.20, 95% CI 0.12-0.33; p < 0.00001).
• Lowering antibiotic use (mean difference -2.65, 95% CI -3.68 to -1.62; p < 0.00001).
• Decreasing the need for rescue medication.
• Reducing absenteeism (mean difference: -2.99, 95% CI -4.03 to -1.95; p < 0.00001).
Therefore, Pidotimod is a viable option for individuals at higher risk for recurrent respiratory infections.[57]
Application in COPD. A recent study in India explored the use of Pidotimod in patients with Chronic Obstructive Pulmonary Disease (COPD), demonstrating its efficacy in managing recurrent respiratory infections. The study also identified certain subgroups of COPD patients who may benefit most from Pidotimod, including:
• Chronic smokers
• Patients with severe COPD, or other comorbidities, particularly those with frequent exacerbations.
All this suggests that Pidotimod can be an effective immunostimulant for managing COPD-related infections.[58]
Pediatric Use. A meta-analysis of 29 randomized controlled trials (RCTs) involving 4344 pediatric patients focused on the use of Pidotimod in the treatment and prevention of recurrent respiratory infections in children. Key findings include:
• Increased proportion of children with fewer respiratory infections (RR 1.59; 95% CI 1.45-1.74; p < 0.00001).
• Reduction in the duration of cough and fever.
• Significant decrease in antibiotic use.
• Improvement in serum levels of immunoglobulins (IgG, IgA, IgM) and T lymphocyte subtypes (CD3+, CD4+).
The results support the use of Pidotimod as an effective therapeutic option for pediatric patients, particularly for reducing recurrent respiratory infections. The treatment also showed benefits in reducing recurrent urinary tract infections in children.
Immunological Effects and Vaccination. Pidotimod may also have a role in improving the efficacy and acceptability of vaccinations. It could enhance the immune response to vaccines, though large-scale studies are still needed to support this use firmly. Elderly individuals, who often have reduced immunological capacity and are at higher risk for infections, might particularly benefit from Pidotimod in combination with vaccines.
Application in Outpatient COVID-19 Management. An exciting area of application for Pidotimod is in outpatient COVID-19 patients. Pidotimod has emerged as a preferred therapy for those who do not qualify for antiviral treatments, based on the quality and number of studies demonstrating its efficacy in reducing inflammatory markers and improving recovery in mild to moderate COVID-19 cases. This positions Pidotimod as a potential immunomodulatory therapy for managing COVID-19, particularly in outpatient settings where antiviral drugs may not be indicated.
Conclusion
Pidotimod has demonstrated significant benefits in treating respiratory infections, particularly in vulnerable populations such as the elderly, children, and COPD patients. It helps enhance immune responses, reduce reinfection rates, lower antibiotic usage, and improve clinical outcomes. While further studies are needed, Pidotimod's potential as an immunomodulatory treatment for conditions like COVID-19 and its ability to improve vaccination responses make it a promising option in various infectious disease settings.References
- Sattler S. The Role of the Immune System Beyond the Fight Against Infection. Adv Exp Med Biol. 2017;1003:3-14. https://doi.org/10.1007/978-3-319-57613-8_1 PMid:28667551
- Liu J, Zhang X, Cheng Y, Cao X. Dendritic cell migration in inflammation and immunity. Cell Mol Immunol. 2021;18(11):2461-71. https://doi.org/10.1038/s41423-021-00726-4 PMid:34302064 PMCid:PMC8298985
- Ucciferri
C, Vecchiet J, Falasca K. Role of monoclonal antibody drugs in the
treatment of COVID-19. World J Clin Cases. 2020;8(19):4280-5. https://doi.org/10.12998/wjcc.v8.i19.4280 PMid:33083387 PMCid:PMC7559676
- Amaya-Uribe
L, Rojas M, Azizi G, Anaya JM, Gershwin ME. Primary immunodeficiency
and autoimmunity: A comprehensive review. J Autoimmun. 2019;99:52-72. https://doi.org/10.1016/j.jaut.2019.01.011 PMid:30795880
- Tuano KS, Seth N, Chinen J. Secondary immunodeficiencies: An overview. Ann Allergy Asthma Immunol. 2021;127(6):617-26. https://doi.org/10.1016/j.anai.2021.08.413 PMid:34481993
- Spotti
D, Biffi M, Coppi G, Silingardi S, Mailland F. Pharmacokinetics of
pidotimod in elderly volunteers and in renal failure patients.
Arzneimittelforschung. 1994;44(12A):1470-2.
- Mailland
F, Coppi G, Silingardi S. Pharmacokinetics and oral bioavailability of
pidotimod in humans. Arzneimittelforschung. 1994;44(12A):1465-9.
- Migliorati
G, D'Adamio L, Coppi G, Nicoletti I, Riccardi C. Pidotimod stimulates
natural killer cell activity and inhibits thymocyte cell death.
Immunopharmacol Immunotoxicol. 1992;14(4):737-48. https://doi.org/10.3109/08923979209009231 PMid:1294620
- Oddera
S, Silvestri M, Sacco O, Eftimiadi C, Rossi GA. [Effect of pidotimod on
phagocytosis and intracellular killing of Staphylococcus aureus by
human circulating polymorphonuclear neutrophils and alveolar
macrophages]. Drugs Exp Clin Res. 1993;19 Suppl:27-35.
- Giagulli
C, Noerder M, Avolio M, Becker PD, Fiorentini S, Guzman CA, et al.
Pidotimod promotes functional maturation of dendritic cells and
displays adjuvant properties at the nasal mucosa level. Int
Immunopharmacol. 2009;9(12):1366-73. https://doi.org/10.1016/j.intimp.2009.08.010 PMid:19712757
- Rossi
GA, Peri C, Raynal ME, Defilippi AC, Risso FM, Schenone G, et al.
Naturally occurring immune response against bacteria commonly involved
in upper respiratory tract infections: analysis of the antigen-specific
salivary IgA levels. Immunol Lett. 2003;86(1):85-91. https://doi.org/10.1016/S0165-2478(02)00290-0 PMid:12600750
- Caccuri
F, Bugatti A, Corbellini S, Roversi S, Zani A, Mazzuca P, et al. The
Synthetic Dipeptide Pidotimod Shows a Chemokine-Like Activity through
CXC Chemokine Receptor 3 (CXCR3). Int J Mol Sci. 2019;20(21). https://doi.org/10.3390/ijms20215287 PMid:31653015 PMCid:PMC6862300
- Wu
T, Cui J, Gao J, Zhou H, Li A, Guo W. Pidotimod enhanced the
anti-growth effect of cisplatin on lung cancer in mice via promoting
anti-tumor immune response. Biochem Biophys Res Commun.
2020;528(4):678-84. https://doi.org/10.1016/j.bbrc.2020.05.117 PMid:32513535
- Zhang
W, Huang J, Liu H, Wen X, Zheng Q, Li L. Whether Immunostimulants Are
Effective in Susceptible Children Suffering From Recurrent Respiratory
Tract Infections: A Modeling Analysis Based on Literature Aggregate
Data. J Clin Pharmacol. 2022;62(2):245-53. https://doi.org/10.1002/jcph.1969 PMid:34535904
- Mahashur
A, Thomas PK, Mehta P, Nivangune K, Muchhala S, Jain R. Pidotimod:
In-depth review of current evidence. Lung India. 2019;36(5):422-33. https://doi.org/10.4103/lungindia.lungindia_39_19 PMid:31464215 PMCid:PMC6710962
- Ciprandi G, Marseglia GL. Pidotimod in pediatrics: new evidence and future perspectives. Multidiscip Respir Med. 2024;19(1). https://doi.org/10.5826/mrm.2024.986
- Clemente
E, Solli R, Mei V, Cera R, Caramia G, Carnelli V, et al. Therapeutic
efficacy and safety of pidotimod in the treatment of urinary tract
infections in children. Arzneimittelforschung. 1994;44(12A):1490-4.
- Buendia
JA, Guerrero Patino D, Lindarte EF. Podotimod in pediatric recurrent
respiratory tract infections: a cost-utility analysis. BMC Pulm Med.
2022;22(1):244. https://doi.org/10.1186/s12890-022-02029-4 PMid:35739542 PMCid:PMC9219210
- Hu
R, Jin C, Lin X, Chen Y, Wang Y, Guo Y. Analysis of Factors and
T-Lymphocyte Subset Changes in Pediatric Recurrent Respiratory
Infections Post-Pidotimod Treatment. Altern Ther Health Med.
2025;31(1):470-5.
- Licari
A, De Amici M, Nigrisoli S, Marseglia A, Caimmi S, Artusio L, et al.
Pidotimod may prevent recurrent respiratory infections in children.
Minerva Pediatr. 2014;66(5):363-7.
- Kulashree
Charusheila Pramod Walavalkar MJ, Mahendra Kelkar, Suhas Kulkarni,
Vijay Tuteja, Francesco Scarci. Efficacy and safety of pidotimod as
adjuvant in the treatment of recurrent upper respiratory tract
infections (URTI) in children. Trends in Medicine 2016; 14
((2)):11-6.
- Namazova-Baranova
LS, Alekseeva AA, Kharit SM, Kozhevnikova TN, Taranushenko TE,
Tuzankina IA, et al. Efficacy and safety of pidotimod in the prevention
of recurrent respiratory infections in children: a multicentre study.
Int J Immunopathol Pharmacol. 2014;27(3):413-9. https://doi.org/10.1177/039463201402700311 PMid:25280032
- Li
X, Li Q, Wang X, Lu M, Shen J, Meng Q. Pidotimod in the treatment of
pediatric recurrent respiratory tract infection. Pak J Med Sci.
2019;35(4):981-6. https://doi.org/10.12669/pjms.35.4.82 PMid:31372128 PMCid:PMC6659081
- Niu
H, Wang R, Jia YT, Cai Y. Pidotimod, an immunostimulant in pediatric
recurrent respiratory tract infections: A meta-analysis of randomized
controlled trials. Int Immunopharmacol. 2019;67:35-45. https://doi.org/10.1016/j.intimp.2018.11.043 PMid:30530167
- La
Mantia I, Grillo C, Mattina T, Zaccone P, Xiang M, Di Mauro M, et al.
Prophylaxis with the novel immunomodulator pidotimod reduces the
frequency and severity of upper respiratory tract infections in
children with Down's syndrome. J Chemother. 1999;11(2):126-30. https://doi.org/10.1179/joc.1999.11.2.126 PMid:10326743
- Valentini
D, Di Camillo C, Mirante N, Marcellini V, Carsetti R, Villani A.
Effects of Pidotimod on recurrent respiratory infections in children
with Down syndrome: a retrospective Italian study. Ital J Pediatr.
2020;46(1):31. https://doi.org/10.1186/s13052-020-0797-5 PMid:32164747 PMCid:PMC7068926
- Zuccotti
GV, Mameli C, Trabattoni D, Beretta S, Biasin M, Guazzarotti L, et al.
Immunomodulating activity of Pidotimod in children with Down syndrome.
J Biol Regul Homeost Agents. 2013;27(1):253-8.
- Manti
S, Filosco F, Parisi GF, Finocchiaro GG, Papale M, Giugno A, et al.
Proposal for a new therapeutic high dosage of Pidotimod in children
with periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA)
syndrome: a randomized controlled study. Ital J Pediatr.
2020;46(1):106. https://doi.org/10.1186/s13052-020-00871-y PMid:32711565 PMCid:PMC7382793
- Esposito
S, Garziano M, Rainone V, Trabattoni D, Biasin M, Senatore L, et al.
Immunomodulatory activity of pidotimod administered with standard
antibiotic therapy in children hospitalized for community-acquired
pneumonia. J Transl Med. 2015;13:288. https://doi.org/10.1186/s12967-015-0649-z PMid:26335787 PMCid:PMC4559022
- Shi
H, Lan L, Lv X, Sun L. Effect of pidotimod combined with azithromycin
on children with mycoplasma pneumonia and the expression levels of
IL-10 and G-CSF in serum. Exp Ther Med. 2019;18(3):1800-6. https://doi.org/10.3892/etm.2019.7725 PMid:31410140 PMCid:PMC6676206
- Gourgiotis
D, Papadopoulos NG, Bossios A, Zamanis P, Saxoni-Papageorgiou P. Immune
modulator pidotimod decreases the in vitro expression of CD30 in
peripheral blood mononuclear cells of atopic asthmatic and normal
children. J Asthma. 2004;41(3):285-7. https://doi.org/10.1081/JAS-120026085 PMid:15260461
- Hejian
CHEN YH, Lingling FU. Effect of pidotimod combined with montelukast
sodium in the treatment of patients with asthma and its influence on
the inflammatory factors and immune function. Chinese Journal of
Primary Medicine and Pharmacy. 2020;12:2885-9.
- Vargas
Correa JB, Espinosa Morales S, Bolanos Ancona JC, Farfan Ale JA.
[Pidotimod in recurring respiratory infection in children with allergic
rhinitis, asthma, or both conditions]. Rev Alerg Mex.
2002;49(2):27-32.
- Ferrario BE, Garuti S, Braido F, Canonica GW. Pidotimod: the state of art. Clin Mol Allergy. 2015;13(1):8. https://doi.org/10.1186/s12948-015-0012-1 PMid:25999796 PMCid:PMC4440502
- Sun
LX YX, Zhang DJ, Wang Y, Wang YJ, Li CG. Influence of pidotimod on the
IL-16, immunoglobulin and T cell subsets in asthmatic children. J Clin
Pediatr 2011;29:777-9.
- Carta
S, Silvestri M, Rossi GA. Modulation of airway epithelial cell
functions by Pidotimod: NF-kB cytoplasmatic expression and its nuclear
translocation are associated with an increased TLR-2 expression. Ital J
Pediatr. 2013;39:29. https://doi.org/10.1186/1824-7288-39-29 PMid:23663325 PMCid:PMC3733658
- Brindisi
G, Zicari AM, Schiavi L, Gori A, Conte MP, Marazzato M, et al. Efficacy
of Pidotimod use in treating allergic rhinitis in a pediatric
population. Ital J Pediatr. 2020;46(1):93. https://doi.org/10.1186/s13052-020-00859-8 PMid:32635938 PMCid:PMC7341603
- Marseglia
GL, Gelardi M, Santus P, Ciprandi G. Reappraisal of Pidotimod: an
immunomodulatory agent with 30-year evidence. Minerva Med.
2024;115(4):503-15. https://doi.org/10.23736/S0026-4806.24.09391-1 PMid:39016527
- Kedia Yash Sanjay IP, Rathi Vidushi. Pidotimod - Current Role and Evidence. Journal of Advanced Lung Health. 2024;5(1):4-7. https://doi.org/10.4103/jalh.jalh_36_24
- Ciaccia A. Pidotimod activity against chronic bronchitis exacerbations. Arzneimittelforschung. 1994;44(12A):1516-20.
- Bisetti
A, Ciappi G, Bariffi F, Catena E, Rocco V, Vaccaro L, et al. Evaluation
of the efficacy of pidotimod in the exacerbations in patients affected
with chronic bronchitis. Arzneimittelforschung.
1994;44(12A):1499-502.
- Benetti
GP, Illeni MT, Passera A, Bombelli G, Lavecchia G, Uslenghi C. Ex vivo
evaluation of pidotimod activity in patients with chronic obstructive
pulmonary disease. Arzneimittelforschung. 1994;44(12A):1503-5.
- Trabattoni
D, Clerici M, Centanni S, Mantero M, Garziano M, Blasi F.
Immunomodulatory effects of pidotimod in adults with community-acquired
pneumonia undergoing standard antibiotic therapy. Pulm Pharmacol Ther.
2017;44:24-9. https://doi.org/10.1016/j.pupt.2017.03.005 PMid:28302543
- Xu
L, Zhao Y, Wu S, Song Q, Ouyang Z, Zhang X, et al. Effects of adjuvant
pidotimod therapy on levels of inflammatory factors and expressions of
serum GM-CSF and KL-6 in elderly patients with mycoplasma pneumonia. Am
J Transl Res. 2021;13(10):11899-907.
- D'Amato
M, Paris D, Molino A, Cuomo P, Fulgione A, Sorrentino N, et al. The
Immune-Modulator Pidotimod Affects the Metabolic Profile of Exhaled
Breath Condensate in Bronchiectatic Patients: A Metabolomics Pilot
Study. Front Pharmacol. 2019;10:1115. https://doi.org/10.3389/fphar.2019.01115 PMid:31632269 PMCid:PMC6785784
- Marogna
M, Ciprandi G. Pidotimod as add-on therapy in patients with
pollen-induced allergic rhinitis and asthma and associated respiratory
infections. J Biol Regul Homeost Agents. 2021;35(3):1053-8. https://doi.org/10.23812/21-103-L PMid:34134476
- Ucciferri
C, Falasca K, Reale M, Tamburro M, Auricchio A, Vignale F, et al.
Pidotimod and Immunological Activation in Individuals Infected with
HIV. Curr HIV Res. 2021;19(3):260-8. https://doi.org/10.2174/1570162X18666210111102046 PMid:33430735
- Larenas-Linnemann
D, Rodriguez-Perez N, Arias-Cruz A, Blandon-Vijil MV, Del Rio-Navarro
BE, Estrada-Cardona A, et al. Enhancing innate immunity against virus
in times of COVID-19: Trying to untangle facts from fictions. World
Allergy Organ J. 2020;13(11):100476. https://doi.org/10.1016/j.waojou.2020.100476 PMid:33072240 PMCid:PMC7546230
- Chatterjee AN, Al Basir F. A Model for SARS-CoV-2 Infection with Treatment. Comput Math Methods Med. 2020;2020:1352982. https://doi.org/10.1101/2020.04.24.20077958
- Ucciferri
C, Barone M, Vecchiet J, Falasca K. Pidotimod in Paucisymptomatic
SARS-CoV2 Infected Patients. Mediterr J Hematol Infect Dis.
2020;12(1):e2020048. https://doi.org/10.4084/mjhid.2020.048 PMid:32670526 PMCid:PMC7340237
- Pradyut
Waghray GJ, Purva Thatai, Vamsi Krishna Kolukula. Efficacy and Safety
of Pidotimod in SARS-CoV-2 Management: A Real-world Evidence Study.
International Journal of Clinical Skills 2021;15(9):510-7.
- Ucciferri
C, Di Gasbarro A, Borrelli P, Di Nicola M, Vecchiet J, Falasca K. New
Therapeutic Options in Mild Moderate COVID-19 Outpatients.
Microorganisms. 2022;10(11). https://doi.org/10.3390/microorganisms10112131 PMid:36363723 PMCid:PMC9697915
- Santus
P, Radovanovic D, Garziano M, Pini S, Croce G, Fuccia G, et al.
Anti-Inflammatory Effects of Immunostimulation in Patients with
COVID-19 Pneumonia. J Clin Med. 2021;10(24). https://doi.org/10.3390/jcm10245765 PMid:34945060 PMCid:PMC8706211
- Ucciferri
C, Moffa L, Moffa S, Vecchiet J, Falasca K. Are monoclonal antibodies
effective in patients with severe obesity in SARS-CoV-2 infected? Immun
Inflamm Dis. 2023;11(2):e771. https://doi.org/10.1002/iid3.771 PMid:36840489 PMCid:PMC9910163
- Cogo R. Pidotimod activity in patients affected by COPD. Minerva Pneumol. 2014;53:21-6.
- Ucciferri
C, Vecchiet J, Auricchio A, Falasca K. Improving BNT162b2 mRNA Vaccine
Tolerability without Efficacy Loss by Pidotimod Supplementation.
Mediterr J Hematol Infect Dis. 2022;14(1):e2022023. https://doi.org/10.4084/MJHID.2022.023 PMid:35444768 PMCid:PMC8992614
- Zhao
N, Liu C, Zhu C, Dong X, Liu X. Pidotimod: a review of its
pharmacological features and clinical effectiveness in respiratory
tract infections. Expert Rev Anti Infect Ther. 2019;17(10):803-18. https://doi.org/10.1080/14787210.2019.1679118 PMid:31603361
- Mahashur
A, Vora A, Waghray P, Jafrey SZ, Karadkhele A, Muchhala S, et al.
Expert Opinion on Usage of Pidotimod in Adult Patients with Chronic
Obstructive Pulmonary Disease: An Indian Perspective. J Assoc
Physicians India. 2021;69(9):11-2.