BsAbs are monoclonal antibodies designed with two fragment antigen-binding (Fab) arms capable of creating an immune synapsis between a T-cell receptor (CD3) and a tumor cell antigen, thus leading to T-cell activation without MHC restriction. As of today, almost all BsAbs contain a fragment-crystallizable (Fc) domain that adds stability, increases the half-life of the molecule, and induces T-cell- and complement-dependent cytotoxicity. At present, BsAbs for MM are targeting BCMA, GPRC5D and the Fc receptor-like 5 (FcRL5).[3]
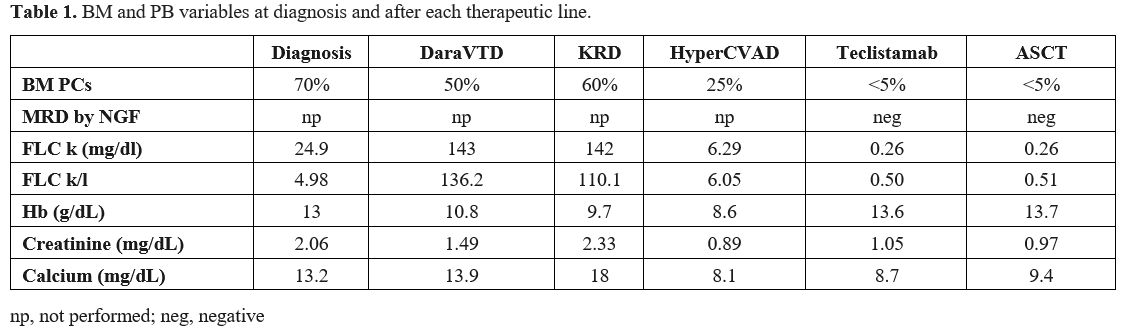
In December 2022, a 52-year-old man presented to our Emergency Department (ED) for pelvis pain, asthenia, polydipsia, vomiting, and fever. Laboratory investigations revealed acute renal failure, hypercalcemia, and altered free light chain levels (FLC-kappa 24.9, FLC-lambda 5, ratio 4.98) with no immunoglobulin heavy chain expression on immunofixation. A CT of the pelvis showed the presence of a large osteolytic lesion. A bone marrow (BM) biopsy confirmed the diagnosis of light chain MM (BM plasma cells (PCs) 70%), and fluorescent in situ hybridization (FISH) revealed the presence of t(11;14) rearrangement, trisomy of chromosome 11 and chromosome 14. According to the Revised International Staging Systema (R-ISS), our patient had stage II MM as he presented with elevated serum β2 microglobulin (β2M), serum albumin, and LDH in range and standard risk cytogenetic. Induction therapy with DaraVTD (daratumumab, bortezomib, thalidomide, dexamethasone) was started, and zoledronic acid was administered monthly. In March 2023, after the fourth cycle, BM aspirate showed disease persistence (BM PCs 50%), FLC assay demonstrated progressive disease, and the patient returned to ED for reoccurrence of acute renal failure and symptomatic hypercalcemia. After stabilization, second-line therapy with the KRd scheme (carfilzomib, lenalidomide, dexamethasone) was initiated. However, treatment was discontinued during the second cycle due to COVID infection. After negativization, the patient was referred to the Nephrology Unit for reoccurrence of hypercalcemia and acute renal failure, which required dialysis. A new BM aspirate revealed 60% of PCs, and FISH was repeated, demonstrating the occurrence of del(17p) and chromosome 1q gain in addition to the known chromosomal abnormalities present at diagnosis. Therefore, we decided to request teclistamab as salvage therapy.
Meanwhile, in June 2023, the Hyper-CVAD scheme was administered since our patient was symptomatic and teclistamab was not immediately available. Renal function improved, and the patient achieved partial response. However, in July 2023, we decided to start teclistamab due to the high risk of progression as our patient presented FHR MM and high-risk cytogenetics. Grade 3 cytokine release syndrome (CRS) occurred after the first step-up dose but rapidly resolved after tocilizumab administration. Teclistamab was then readministered with no further complications, and one month later, our patient was in stringent complete response (sCR) with negative minimal residual disease (MRD) by next-generation flow (NGF) on BM aspirate. Teclistamab was temporarily discontinued to allow the collection of peripheral blood stem cells (PBSC) after mobilization with only G-CSF (7 x 106 CD34+ cells/kg harvested). High-dose melphalan conditioning followed by ASCT was performed in September 2023; grade 3 infection occurred during hospitalization but promptly recovered with IV antibiotics. In December 2023, three months after ASCT, response evaluation confirmed sCR with negative MRD by NGF on BM aspirate. Afterward, in February 2024, we decided to resume teclistamab as a maintenance therapy after ASCT as off-label therapy. Currently, our patient is still healthy and in sCR receiving teclistamab biweekly.
Early identification of FHR in MM remains a clinical challenge since we have no standardized systems to predict FHR in advance. Gene expression profiles, circulating tumor cells, and evaluation of tumor microenvironment are novel MM biomarkers that could better stratify prognosis in MM; however, they still need to be validated in clinical trials.[4]
In our case, the patient presented with R-ISS stage II MM, but he did not present any feature that could help us predict refractoriness to induction treatment. Cytogenetic evaluation at diagnosis by FISH demonstrated three cytogenetic abnormalities, yet none of these were defined as high-risk genetic lesions. Subsequently, after progression during second-line treatment with KRd, new cytogenetic abnormalities were revealed by FISH, including del(17p), which is associated with poor prognosis.[5] Interestingly, our patient turned out to be also a triple-class refractory since he did not respond to proteasome inhibitors, immunomodulatory agents, and anti-CD38 monoclonal antibody.
Correct management of FHR MM still represents an unmet medical need. Treatment intensification with rapid switching drug classes or early use of new immunotherapies, such as CAR-T cells or bispecific antibodies, has been proposed in this subset of patients. Another strategy seems to start treatment promptly in case of MRD resurgence or biochemical relapse. These treatment approaches need to be validated in clinical trials.[4]
Teclistamab is a first-in-class BCMA/CD3 bispecific antibody approved for the treatment of patients with relapsed/refractory multiple myeloma. The approval of teclistamab was based on the results from the MajesTEC-1 trial. In this phase 1/2 pivotal clinical study, patients had previously received at least three therapy lines, and most of them (77.6%) were triple-class refractory. At least one high-risk cytogenetic alteration was present in a quarter of patients (25.7%). After a median follow-up of 14.1 months, the trial showed a high rate of deep response with a median progression-free survival of 11.3 months and a median overall survival of 18.3 months.[6] Better responses were seen in patients who received no more than 3 lines of therapy; a recent MajesTEC-1 correlative analysis demonstrated that non-responder patients presented parameters associated with T-cell exhaustion. Indeed, the use of teclistamab in earlier lines of therapy could produce deeper responses, as T-cell exhaustion is often associated with heavily pre-treated patients.[7]
In conclusion, teclistamab proved to be effective in an FHR and triple-class refractory MM patient. Of note, teclistamab did not limit mobilization and collection of PBSC and ASCT was performed after teclistamab temporary discontinuation, however further studies are necessary to validate this approach.
Authorship Contributions
VS conceived and wrote the manuscript. VS, SC, BEV and CC collected and analyzed clinical data. MB and AG conceived, revised, and finally approved the manuscript. All authors approved the final manuscript.Ethical Approval
Patient gave written consent for his case to be published.References
- Banerjee R, Cicero KI, Lee SS, Cowan AJ. Definers
and drivers of functional high-risk multiple myeloma: insights from
genomic, transcriptomic, and immune profiling. Front Oncol. 2023 Oct
2;13:1240966. https://doi.org/10.3389/fonc.2023.1240966 PMid:37849816 PMCid:PMC10577204
- Sammartano
V, Franceschini M, Fredducci S, Caroni F, Ciofini S, Pacelli P, Bocchia
M, Gozzetti A. Anti-BCMA novel therapies for multiple myeloma. Cancer
Drug Resist. 2023 Mar 22;6(1):169-181. https://doi.org/10.20517/cdr.2022.138 PMid:37065871 PMCid:PMC10099603
- Puppi
M., Sacchetti I., Mancuso K., Tacchetti P., Pantani L., Rizzello I.,
Iezza M., Talarico M., Manzato E., Masci S., Restuccia R., Barbato S.,
Armuzzi S., Taurisano B., Vigliotta I., Zamagni E. Bispecific
antibodies and CART in multiple myeloma: appropriate selection of
patients and sequencing. Mediterr J Hematol Infect Dis 2025, 17(1):
e2025045 https://doi.org/10.4084/MJHID.2025.045 PMid:40375902 PMCid:PMC12081044
- Gay
F, Bertuglia G, Mina R. A rational approach to functional high-risk
myeloma. Hematology Am Soc Hematol Educ Program. 2023 Dec
8;2023(1):433-442. https://doi.org/10.1182/hematology.2023000443 PMid:38066896 PMCid:PMC10727111
- Rajkumar
SV. Multiple myeloma: 2024 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2024 Sep;99(9):1802-1824. https://doi.org/10.1002/ajh.27422 PMid:38943315
- Moreau
P, Garfall AL, van de Donk NWCJ, Nahi H, San-Miguel JF, Oriol A, Nooka
AK, Martin T, Rosinol L, Chari A, Karlin L, Benboubker L, Mateos MV,
Bahlis N, Popat R, Besemer B, Martínez-López J, Sidana S, Delforge M,
Pei L, Trancucci D, Verona R, Girgis S, Lin SXW, Olyslager Y, Jaffe M,
Uhlar C, Stephenson T, Van Rampelbergh R, Banerjee A, Goldberg JD,
Kobos R, Krishnan A, Usmani SZ. Teclistamab in Relapsed or Refractory
Multiple Myeloma. N Engl J Med. 2022 Aug 11;387(6):495-505. https://doi.org/10.1056/NEJMoa2203478 PMid:35661166 PMCid:PMC10587778
- van
de Donk N, Cortes-Selva D, Casneuf T, Vishwamitra D, Stein S, Perova T,
Ramos E, Van Steenbergen L, Boominathan R, Lau O, Davis C, Banerjee A,
Stephenson T, Uhlar C, Kobos R, Goldberg J, Pei L, Trancucci D, Girgis
S, Lin SXW, Wu LS, Moreau P, Usmani S, Bahlis NJ, Verona R.
P32 MAJESTEC-1: CORRELATIVE ANALYSES OF Teclistamab, a b-cell
maturation antigen (bcma) x cd3 bispecific antibody, in patients with
relapsed/refractory multiple myeloma (rrmm). Hemasphere. 2023 May
9;7(Suppl):28-29. https://doi.org/10.1097/01.HS9.0000936256.84340.a7 PMCid:PMC10171470