Since the Italian Drug Agency approved the combination of Dara-VTD as induction before and consolidation after autologous stem cell transplantation (ASCT) in NDTE MM patients at the end of 2021, we retrospectively analyzed the data of the 66 patients consecutively treated with this combination in a real-life setting at the ASST Papa Giovanni XXIII, Bergamo, Italy, between January 2022 and December 2023. The aims were to describe data on treatment response, toxicity, HSC mobilization and collection, progression-free survival (PFS), and overall survival (OS) and to compare these data with those of a historical group of 76 NDTE MM patients consecutively treated with the VTD combination between January 2019 and December 2021.
The baseline characteristics were similar in the Dara-VTD and VTD groups in terms of age distribution (median age 61 years, range 42-71 vs median age 63 years, range 33-73, respectively). Similarly, the prevalences of anemia (30% vs. 22%), kidney failure (14% vs. 5%), hypercalcemia (9% vs 12%), skeletal involvement (76% vs. 82%), and bone-marrow plasma-cell infiltration > 60% (61% vs 47%) at the start of treatment were similar in the Dara-VTD and VTD groups, respectively. The two groups showed a similar proportion of high-risk cytogenetic abnormalities (30% vs 39% in the Dara-VTD and VTD group, respectively); cytogenetic data were not available for a proportion of patients in both groups (29% vs 18%, respectively), mainly due to the failure of plasma cell enrichment in bone marrow samples, required for fluorescence in situ hybridization (FISH) analysis. Also, the prognostic scores Durie and Salmon, International Staging System, Revised ISS (R-ISS)[4] and Revised 2 ISS (R2-ISS)[5] did not show significant differences between the two groups (data not shown). The only exception was a higher rate of extramedullary disease in the Dara-VTD group (N=6 vs. N=1 in the VTD group; p=0.0497), which was mostly accounted for by a higher number of plasma cell leukemia (PCL) cases. This difference is likely due to the increasing use of immunophenotypic analysis - a much more sensitive technique compared to the peripheral blood smear morphological analysis - to detect circulating plasma cells at the time of MM diagnosis. Also, the new diagnostic criteria for PCL, recently revised from 20% to ≥5% of circulating plasma cells in peripheral blood, have resulted in a less restrictive definition of PCL.[6,7]
Figure 1 summarizes the patients’ flow during the different treatment phases; the most common reason for definitive discontinuation was progressive disease in both groups. During the induction and consolidation therapy, the proportion of patients who reduced the number and/or posology of one or more drugs was significantly higher in the Dara-VTD group, because of clinical choices secondary to preexistent comorbidities or due to the occurrence of adverse events (AEs) (Table 1). Among the most common AEs, the proportion of patients who developed grade 3 or 4 neutropenia requiring G-CSF (granulocyte colony-stimulating factor) was significantly higher in the Dara-VTD group (N=14, 21.2%) than in the VTD group (N=5, 6.6%) (p=0.01). Furthermore, daratumumab addition was associated with a significantly higher incidence of symptomatic hypogammaglobulinemia (defined as serum immunoglobulin levels ≤400 mg/dL and recurrent infections) requiring monthly supplementation of intravenous immunoglobulins (N=10, 15.2% vs. N=4, 5.3% in the VTD group; p=0.049), even though it did not cause a higher incidence of grade 3 or 4 infections. We observed the occurrence of grade 3 or 4 hepatotoxicity more frequently in the Dara-VTD group (N=11, 16.7% vs. N=3, 3.9% in the VTD group; p=0.02); this was characterized by an isolated increase of transaminases or gamma-glutamyl transferase, without reactivation of hepatitis B or C viruses or pathological findings by abdominal echography. This toxicity recovered after reducing the posology of one or more drugs or temporarily interrupting the therapy and did not require liver biopsy in any patient. At present, the pathophysiology of daratumumab-related hepatotoxicity is not understood, although CD38 hepatocyte intranuclear expression has been recently described.[8] In particular, whether daratumumab per se or its combination with one or more of the other drugs increases the risk of hepatotoxicity remains to be established.
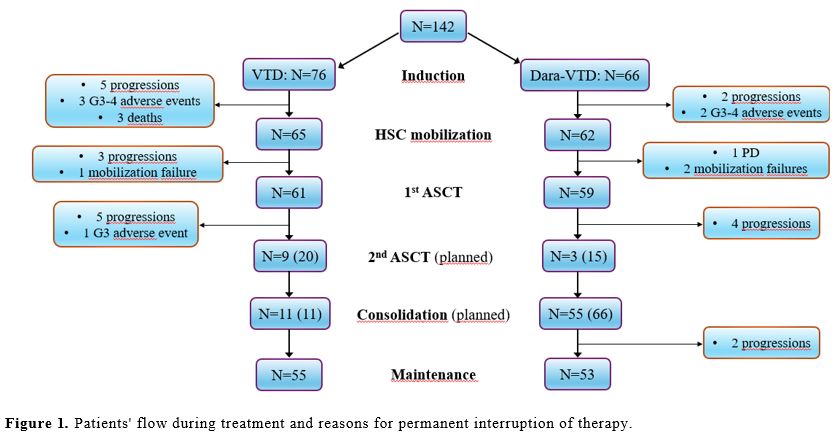 |
Figure 1. Patients' flow during treatment and reasons for permanent interruption of therapy. |
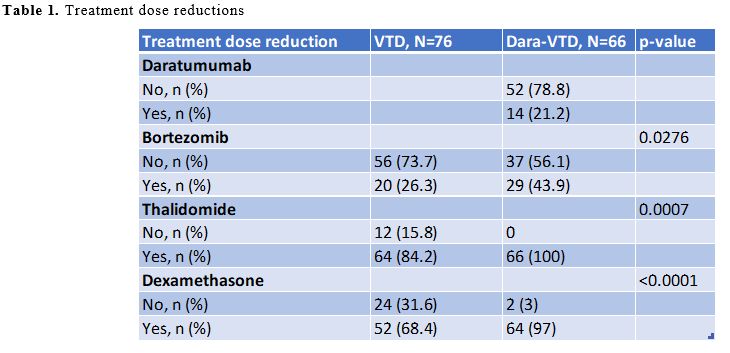 |
Table 1. Treatment dose reductions. |
Our data confirmed the negative impact of daratumumab on HSC mobilization and collection. The proportion of poor mobilizers (defined as having a number of CD34+ cells ≤20/mmc in peripheral blood on the 11th day after cyclophosphamide or, for patients receiving G-CSF only, on the fourth day of G-CSF administration) was significantly higher in the Dara-VTD group (N=27, 43.5%) than in the VTD group (N=6, 9.2%) (p<0.0001), which led to a more frequent administration of plerixafor in the Dara-VTD group (N=32, 51.6% vs. N=5, 7.7%; p<0.0001). The median number of harvested HSC was significantly lower in the Dara-VTD group (4.2x106/kg, range 1.3-11) compared to the VTD group (6.5x106/kg, range 2.6-14.9) (p<0.0001). Nevertheless, the number of harvest failures was low in both groups (N=2 in the Dara-VTD group vs N=1 in the VTD group). To reduce the risk of harvest failure, we increased the range of days between the last administration of thalidomide and the mobilization in the Dara-VTD group up to 8-157 (median 28 days) compared to the 7-178 days (median 19 days) of the VTD group (p=0.0088). Despite the lower number of collected HSC, almost all patients were able to undergo the ASCT (Figure 1). Approximately two-thirds of the patients in both groups received a full dose of melphalan (i.e., 200 mg/sm), and the other third required a reduction to 100 or 140 mg/sm because of kidney failure or patients’ unfitness. Only 3 out of the 15 high-risk patients in the Dara-VTD for whom a 2nd ASCT had been planned could undergo this procedure, mainly because of the small number of harvested HSC. Three cases of grade 5 non-hematological AEs occurred during the treatment in the VTD group (2 Covid pneumonia, during the first and most severe pandemic wave, and 1 cardiac failure), while there were no grade 5 AEs in the Dara-VTD group.
Unlike the CASSIOPEIA trial, in our experience, the rates of optimal response (defined as very good partial response or CR) were not significantly increased in the Dara-VTD group compared to the VTD group (77.3% vs 76.3%, respectively) by the end of the consolidation phase. This apparently disappointing result may be explained by the small number of patients in the two groups and may have also been biased by the fact that, outside of clinical trials, we do not routinely analyze bone marrow for morphology and MRD status at the end of each treatment phase. Nevertheless, the response rate improved through the different phases of treatment and reached a remarkable rate of 75.9% of optimal response among the 29 out of the 66 (43.9%) Dara-VTD group patients who had clinical or disease-related features that would have made them ineligible for the CASSIOPEIA trial (i.e., age >65 years, Eastern Cooperative Oncology Group performance status >2, hemoglobin <7.5 g/dL, renal dysfunction and/or corrected serum calcium >14 mg/dL). As a comparison, the other 37 patients of the Dara-VTD group showed an optimal response rate of 78.4% by the end of the consolidation phase.
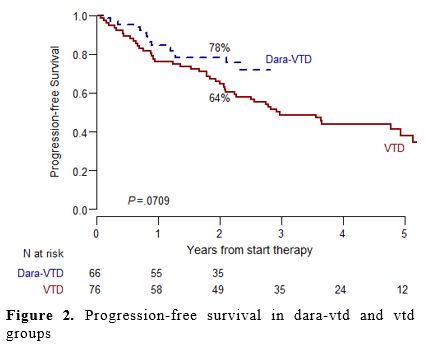
With a median follow-up of 2.1 years for the Dara-VTD group and 4.1 years for the VTD group, the 2-year PFS was higher in the Dara-VTD group compared to the VTD group (78% vs. 64%, respectively), with a trend to statistical significance (p=0.07) (Figure 2). The superimposable rates of 2-year OS in the two groups (87% in both groups) possibly underline the effectiveness of the subsequent available lines of therapy (data not shown).
The principal limitations of this study are its retrospective nature, the small sample size, and the short follow-up; on the other hand, the strengths are represented by the superimposable baseline characteristics of the two groups, allowing their comparison, and the considerable proportion of patients with features of frailty and/or aggressive disease.
In conclusion, this real-life experience confirms the effectiveness of the Dara-VTD combination with a manageable toxicity profile. To our knowledge, these results describe for the first time the hepatotoxicity secondary to this treatment scheme, mostly reversible and of unknown pathophysiology. Our data also confirm the negative impact of daratumumab on HSC harvest, being associated with a higher incidence of poor mobilizers, a more frequent administration of plerixafor, and a lower number of harvested HSC compared to VTD; nevertheless, almost all patients were able to undergo a 1st ASCT. Only a minority of high-risk patients could receive a 2nd ASCT in the Dara-VTD group, whose role in a modern first-line therapy based on quadruplets in induction and post-ASCT consolidation should be further investigated.
References
- Moreau, P. et al. Bortezomib, thalidomide, and
dexamethasone with or without daratumumab before and after autologous
stem-cell transplantation for newly diagnosed multiple myeloma
(CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 394,
29-38 (2019). https://doi.org/10.1016/S0140-6736(19)31240-1 PMid:31171419
- Eleutherakis
Papaiakovou, E. et al. Impact of daratumumab on stem cell mobilization
and collection, engraftment and early post-transplant complications
among multiple myeloma patients undergoing autologous stem cell
transplantation. Leuk Lymphoma 64, 2140-2147 (2023). https://doi.org/10.1080/10428194.2023.2253479 PMid:37655597
- Cavallaro,
G. et al. Impact of the Addition of Daratumumab to the Standard
Bortezomib-Thalidomide-Dexamethasone Regimen on Hematopoietic Stem Cell
Mobilization and Collection, Post-Transplant Engraftment and Infectious
Complications: A Case-Control Multicentre Real-Life Analysis. Mediterr
J Hematol Infect Dis 16, e2024049 (2024). https://doi.org/10.4084/MJHID.2024.049 PMid:38882460 PMCid:PMC11178055
- Palumbo,
A. et al. Revised international staging system for multiple myeloma: A
report from international myeloma working group. Journal of Clinical
Oncology 33, 2863-2869 (2015). https://doi.org/10.1200/JCO.2015.61.2267 PMid:26240224 PMCid:PMC4846284
- D'Agostino,
M. et al. Second Revision of the International Staging System (R2-ISS)
for Overall Survival in Multiple Myeloma: A European Myeloma Network
(EMN) Report Within the HARMONY Project. Journal of Clinical Oncology
364, (2022). https://doi.org/10.1200/JCO.21.02614 PMid:35605179
- Fernández
de Larrea, C. et al. Primary plasma cell leukemia: consensus definition
by the International Myeloma Working Group according to peripheral
blood plasma cell percentage. Blood Cancer J 11, (2021).
https://doi.org/10.1038/s41408-021-00587-0 https://doi.org/10.1038/s41408-021-00587-0 PMid:34857730 PMCid:PMC8640034
- Jung, S. H. & Lee, J. J. Update on primary plasma cell leukemia. Blood Res 57, 62 (2022). https://doi.org/10.5045/br.2022.2022033 PMid:35483928 PMCid:PMC9057670
- Rah, S. Y. et al. CD38/ADP-ribose/TRPM2-mediated nuclear Ca2+ signaling is essential for hepatic gluconeogenesis in fasting and diabetes. Exp Mol Med 55, 1492-1505 (2023). https://doi.org/10.1038/s12276-023-01034-9 PMid:37394593 PMCid:PMC10393965