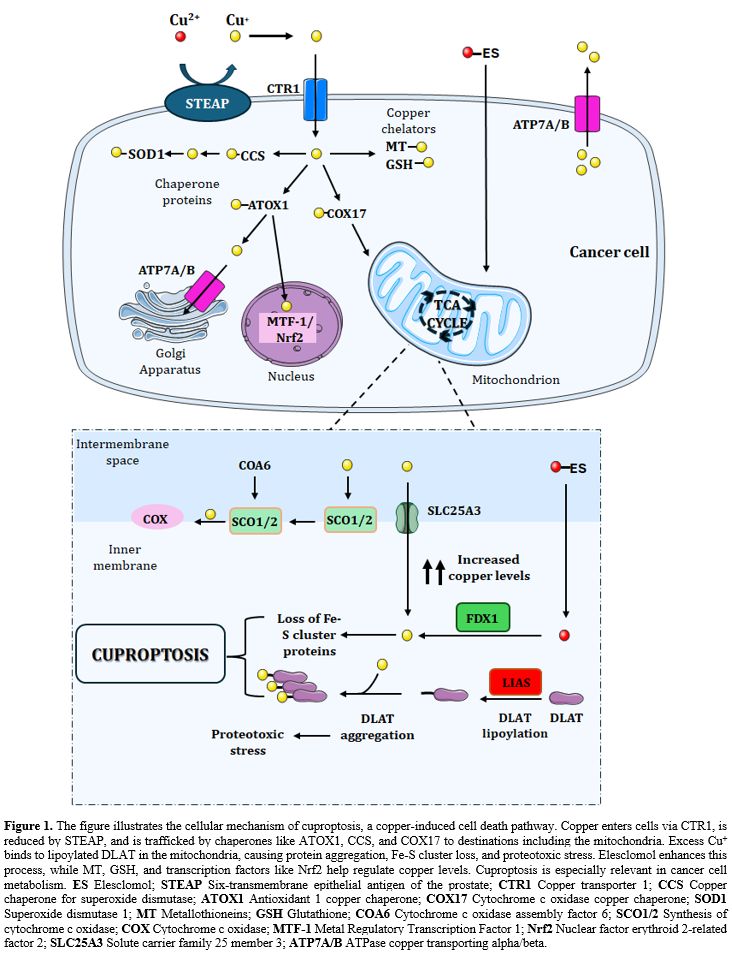
Copper (Cu) is an essential trace element necessary for the proper functioning of various metabolic enzymes involved in numerous critical physiological processes. These include mitochondrial energy production, neurotransmitter and tyrosine metabolism, maintenance of redox balance, and extracellular matrix remodeling. Maintaining systemic copper levels within a narrow optimal range is crucial to prevent either copper deficiency or toxicity.[3-4]
The absorption of dietary copper primarily takes place in the duodenum and small intestine through the copper transport protein 1 (CTR1). The absorption process involves metalloreductases such as STEAP (six-transmembrane epithelial antigen of the prostate) and DCYTB (duodenal cytochrome b), which convert divalent copper (Cu2+) into monovalent copper (Cu+), the ionic form transported by CTR1. After absorption, copper enters the bloodstream, bound to proteins like albumin, transcuprein, histidines, and macroglobulins, and is transported to the liver. Hepatocytes in the liver also utilize CTR1 to facilitate copper uptake. Inside the hepatocytes, copper is either distributed to specific enzymes through specialized chaperone proteins such as ATOX1, CCS, and COX17 or stored by binding to metallothionein (MT).[5-8]
The liver plays a critical role in regulating systemic copper balance by secreting excess copper into bile for elimination through fecal excretion, the primary pathway for removing copper from the body. Other elimination pathways, such as urine, sweat, and menstruation, contribute minimally to copper loss. Copper homeostasis is dynamically maintained through adjustments in intestinal copper absorption and biliary excretion in response to fluctuations in dietary copper intake.
At the cellular level, copper homeostasis involves a complex and precise regulation network consisting of copper transporters, chaperones, and enzymes. The primary cellular copper transporter, CTR1, adjusts its expression according to cellular copper levels — upregulated during copper deficiency and downregulated during copper overload — to regulate copper uptake effectively.
Within cells, copper trafficking and distribution involve specific copper chaperones:
• ATOX1 delivers copper to copper-transporting ATPases (ATP7A and ATP7B), enabling copper incorporation into critical enzymes like ceruloplasmin, tyrosinase, and lysyl oxidase.
• CCS (copper chaperone for superoxide dismutase) transfers copper to superoxide dismutase 1 (SOD1), which is essential for neutralizing reactive oxygen species and maintaining oxidative balance in the cytoplasm and mitochondria.
• COX17 transports copper into the mitochondria, delivering it to mitochondrial chaperones SCO1 and COX11 for assembly into cytochrome c oxidase, a crucial enzyme for mitochondrial oxidative phosphorylation and energy production.
Disruptions or mutations in these copper transporters or chaperone proteins can severely impact copper metabolism, leading to developmental disorders, mitochondrial dysfunction, oxidative stress, and various copper-related diseases.[9-12]
In 2022, a novel form of copper-induced RCD, termed "cuproptosis," was described. This pathway was shown to be mechanistically distinct from previously known forms of cell death, crucially dependent on mitochondrial respiration, and initiated by the direct binding of copper to lipoylated mitochondrial proteins.[3] This discovery opened a new avenue of investigation, particularly relevant to cancer biology, as many cancer cells exhibit altered copper metabolism, often characterized by increased copper uptake and accumulation, potentially creating a specific vulnerability.[3,5] This differential copper handling between normal and malignant cells suggests a potential therapeutic window for selectively targeting cancer cells through the induction of cuproptosis.
This review aims to consolidate and analyze the current understanding of cuproptosis, drawing exclusively from the available scientific publications up to April 2025. The specific objectives are to: (1) describe the reported molecular mechanisms governing cuproptosis; (2) investigate the documented associations between cuproptosis and various solid tumors, focusing on its relevance for prognosis and therapy; (3) examine the reported relationship between cuproptosis and lymphoma; (4) review the described connections between cuproptosis and viral infections; and (5) summarize the therapeutic strategies reported in the literature that aim to modulate cuproptosis for cancer treatment. By synthesizing these findings, this review seeks to provide an organized and comprehensive overview of the state of cuproptosis research as represented in the selected articles, highlighting potential clinical implications and areas warranting further investigation.
Mechanism of Cuproptosis
The reviewed literature characterizes
cuproptosis as a unique cell death pathway initiated by an excess of
intracellular copper, with a defining dependence on mitochondrial respiration
and a direct molecular interaction with specific mitochondrial proteins.[3]
The process hinges on protein lipoylation, a relatively rare post-translational
modification where lipoic acid is covalently attached to specific lysine
residues of mitochondrial enzymes involved primarily in oxidative metabolism.
The central initiating event reported is the binding of excess intracellular
copper, potentially facilitated by reduction from Cu2+ to the more reactive Cu+
state, to the lipoyl moieties of key TCA cycle enzymes.[3] Among these,
dihydrolipoamide S-acetyltransferase (DLAT), a component of the pyruvate
dehydrogenase (PDH) complex, and other lipoylated proteins involved in α-ketoglutarate metabolism are
highlighted as primary targets.[3]
This copper-protein interaction is reported to
cause the abnormal oligomerization and aggregation of these lipoylated proteins
within the mitochondrial matrix. This aggregation event is not merely
structural; it precipitates a cascade of detrimental downstream consequences. A
key reported outcome is the destabilization and subsequent loss of iron-sulfur
(Fe-S) cluster-containing proteins, which are vital for numerous mitochondrial
functions, including electron transport and metabolic catalysis.[2] The
combined effect of lipoylated protein aggregation and Fe-S cluster protein loss
results in profound proteotoxic stress and metabolic catastrophe within the
mitochondria, ultimately leading to cell death (Figure 1).[2,13]
Several key molecular players regulating
sensitivity to cuproptosis have been identified in the reviewed articles.
Ferredoxin 1 (FDX1), a mitochondrial reductase, acts upstream and is essential
for cuproptosis induction, possibly by reducing copper to its more toxic Cu+
form, thereby facilitating its interaction with lipoylated targets.[13]
Consequently, cells lacking FDX1 exhibit resistance to this form of cell death.
Similarly, enzymes involved in the biosynthesis of lipoic acid, such as lipoic
acid synthetase (LIAS), are critical, and their absence also confers resistance.[13,16] Conversely, proteins involved in copper transport and buffering
significantly influence cuproptosis sensitivity. The copper chaperone ATOX1,
for instance, was reported to modulate intracellular copper transport and
influence cuproptosis sensitivity in the context of lymphoma cell proliferation.[17,18] Metallothioneins, cysteine-rich proteins known for their ability to
bind and sequester heavy metals, including copper, act as protective factors.
One study demonstrated that metallothionein could mitigate doxorubicin-induced
cardiomyopathy, a condition associated with mitochondrial dysfunction,
specifically by reducing cuproptosis (Figure 1).[14]
The cellular environment also plays a
regulatory role. Hypoxia-inducible factor-1α (HIF-1α), a master regulator of cellular responses to
low oxygen, was identified as a driver of cancer cell resistance to cuproptosis.[19] This finding suggests that the tumor microenvironment, particularly
oxygen availability, can significantly impact a cell's susceptibility to this
death pathway. Furthermore, mitochondrial integrity and function are central,
as evidenced by studies analyzing mitochondrial alterations and depolarization
signatures in cancers like hepatocellular carcinoma[20] and non-small cell
lung cancer,[21] linking these features to cuproptosis-related genes and
prognosis.
While cuproptosis possesses a distinct
mechanism, evidence from the reviewed literature suggests potential interplay
with other cell death pathways. The redox-active protein High-Mobility Group
Box 1 (HMGB1), known for its role in inflammation and various forms of cell
death, is discussed within the broader context of redox signaling and cell
death, potentially intersecting with the oxidative and proteotoxic stress
characteristic of cuproptosis.[2] Moreover, certain therapeutic interventions
appear capable of activating multiple death programs simultaneously. For
example, the drug disulfiram was reported to induce cell death in endometrioid
epithelial ovarian cancer cells through mechanisms involving both apoptosis and
cuproptosis.[22] This observation is mirrored in the design of novel
nanotherapeutic strategies explicitly aimed at co-inducing cuproptosis
alongside ferroptosis[23] or apoptosis,[24,25] suggesting that leveraging
multiple pathways might offer synergistic advantages in cancer therapy.
Cuproptosis in Solid Tumors
The relationship between cuproptosis and the biology of solid tumors has been a major focus of investigation within the reviewed articles, driven by the frequent observation of altered copper metabolism in cancer cells and the potential for therapeutic exploitation of this phenomenon. Research has spanned various aspects, from identifying prognostic markers to developing novel therapeutic strategies across a diverse range of solid malignancies.Prognostic Significance and Biomarker Development. A recurrent theme across numerous studies is the exploration of cuproptosis-related genes and associated non-coding RNAs, particularly long non-coding RNAs (lncRNAs), as potential biomarkers for predicting patient outcomes in solid tumors. Pan-cancer analyses have systematically profiled the expression of cuproptosis gene sets, revealing widespread dysregulation across different cancer types and suggesting a fundamental role for this pathway in general cancer biology.[26] Building on this, researchers have developed and validated prognostic signatures based on the expression levels of specific cuproptosis-associated genes or lncRNAs in various individual cancer types.
For instance, in gastric cancer, studies have assessed the prognostic value of genes associated with disulfidptosis (a related form of cell death involving disulfide stress, potentially overlapping with copper-induced redox stress)[27] and directly investigated the clinical significance and potential application of cuproptosis-related genes.[28] Furthermore, the prognostic marker Nucleophosmin 1 (NPM1) in gastrointestinal cancers has been linked mechanistically to both m6A RNA modification and cuproptosis pathways, suggesting complex regulatory interactions.[29]
Lung cancer, particularly non-small cell lung cancer (NSCLC) and lung adenocarcinoma (LUAD), has been a frequent subject of such investigations. Several independent research groups have reported the development of prognostic models based on cuproptosis-related lncRNA signatures[22,30,31] or gene expression patterns.[32] These signatures were often found to correlate not only with patient survival but also with characteristics of the tumor microenvironment, such as the infiltration levels of different immune cell types,[32] and potentially with sensitivity to treatments like radiotherapy.[21] An integrative analysis further strengthened this link by associating cuproptosis-related mitochondrial depolarization genes with prognosis in NSCLC.[21] Zhang and colleagues also specifically highlighted the prognostic value and immunological function relevance of cuproptosis-related genes in LUAD.[22]
Similar prognostic investigations have been conducted in other solid tumors. In neuroblastoma, distinct cuproptosis-related molecular subtypes and gene signatures have been identified and associated with the tumor's immunophenotype and patient prognosis.[33] Multi-omics approaches in neuroblastoma have explored how cell death pathways, potentially including cuproptosis, are regulated by other cellular processes like fatty acid metabolism,[23] while single-cell RNA sequencing has provided insights into the tumor microenvironment and cell death-related therapeutic targets within this malignancy.[34] For pancreatic adenocarcinoma, a prognostic model based on cuproptosis-related lncRNAs was proposed, which also aimed to predict the effectiveness of immunotherapy.[35] In colon adenocarcinoma, a novel prognostic signature derived from cuproptosis-related lncRNAs was developed, with reported predictive value for patient response to both immunotherapy and chemotherapy.[36] The broader relevance of regulated cell death pathways, implicitly including cuproptosis, has also been reviewed in the context of head and neck squamous cell carcinoma (HNSCC) development.[1] Even in hematological malignancies like acute myeloid leukemia (AML), comprehensive analyses have explored the predictive value of cuproptosis-associated lncRNAs and their related competing endogenous RNA (ceRNA) networks.[37]
The consistent finding across these diverse solid tumor types that the expression patterns of cuproptosis pathway components correlate with clinical outcomes strongly suggests that this cell death mechanism is fundamentally intertwined with tumor progression and patient survival. This body of work provides a solid foundation for the further development and validation of cuproptosis-related biomarkers for clinical applications in risk stratification and treatment guidance.
Therapeutic Strategies Targeting Cuproptosis in Solid tumors. The potential to selectively eliminate cancer cells by inducing cuproptosis, leveraging their often-altered copper metabolism, has spurred considerable effort in developing therapeutic strategies, as reflected in the reviewed literature. These strategies range from utilizing existing compounds to designing highly sophisticated nanomedicine approaches and combination therapies.
One direct approach involves the use of copper ionophores, molecules that facilitate the transport of copper ions across cellular membranes, thereby increasing intracellular copper concentrations to potentially toxic levels. Elesclomol is a prominent example of such an agent, known to transport copper into mitochondria and effectively trigger cuproptosis.[3,38] A review by Tarin et al. details the discovery, mechanism of action targeting mitochondria, and potential applications of Elesclomol.[39] Drug repositioning offers another avenue; for instance, Disulfiram, an established drug used for treating alcoholism, has been investigated for anti-cancer activity and was reported to induce cell death in endometrioid epithelial ovarian cancer cells through both apoptosis and cuproptosis pathways.[22]
Nanotechnology has emerged as a powerful tool for developing targeted cuproptosis-inducing therapies. Numerous studies report the design and preclinical evaluation of various nanoplatforms engineered to deliver copper or cuproptosis-inducing agents specifically to tumor sites, often incorporating features for controlled release or activation. Examples from the reviewed articles include: Elesclomol encapsulated within copper oxide nanoplatforms;[40] near-infrared light-activatable copper nanoplatforms designed to synergize with chemotherapy prodrugs like 5-azacytidine;[40] metal-phenolic networks tailored to eliminate hypoxic tumor cells by inducing oxidative and proteotoxic stress;[41] cystine-modified lignin-copper coordination nanocarriers intended to enhance tyrosine kinase inhibition via cuproptosis;[42] p-n heterojunction sonosensitizers;[16] dual-responsive biomimetic "cyto-nanos" for precision mitochondrial intervention;[43] bioactive layered double hydroxides;[41] bimetallic iron-copper metal-organic frameworks (MOFs) designed as "cellular Trojan horses";[23] tumor microenvironment-activated immunomodulatory nanosheets loaded with copper(II) and the chemotherapeutic 5-FU;[25] copper-coordinated covalent organic frameworks generating Fenton-like effects;[44] and intelligent cell-derived nanorobots.[45] These diverse nanostrategies often aim to overcome limitations of systemic copper administration by enhancing tumor accumulation and minimizing off-target toxicity, sometimes employing triggers like tumor acidity, hypoxia, or external stimuli (light, ultrasound) for activation.
A significant trend in developing cuproptosis-based therapies is the combination with other treatment modalities to achieve synergistic effects and overcome potential resistance. Synergies have been actively explored with immunotherapy, based on the premise that inducing cuproptosis, particularly if it leads to immunogenic cell death (ICD), can stimulate anti-tumor immune responses. Several nanoplatforms are explicitly designed not only to induce cuproptosis but also to modulate the tumor immune microenvironment or elicit ICD.[16,24,38,41,44] Combination with conventional chemotherapy is another approach, exemplified by nanocarriers co-delivering copper and agents like 5-azacytidine prodrug[40] or 5-FU.[25] Sonodynamic therapy (SDT), which uses ultrasound to activate sonosensitizers and generate cytotoxic reactive oxygen species, has also been combined with cuproptosis induction. Several nanoplatforms described function as sonosensitizers that, upon ultrasound irradiation, trigger both SDT effects and cuproptosis, leading to enhanced tumor killing and potentially improved immune responses.[16,41,43] Furthermore, recognizing the complexity of cell death regulation, strategies are being developed to simultaneously trigger multiple RCD pathways, such as combining cuproptosis with ferroptosis[23] or apoptosis,[22,25] aiming to maximize cancer cell killing and circumvent resistance mechanisms specific to a single pathway.
Understanding and overcoming resistance to cuproptosis induction is critical for successful therapeutic translation. Factors conferring resistance have been identified, including the protective role of metal-binding proteins like metallothioneins[14] and the influence of the tumor microenvironment, particularly hypoxia, which can activate HIF-1α and subsequently drive resistance to cuproptosis.[19] Developing strategies to counteract these resistance mechanisms will be essential for the clinical success of cuproptosis-targeting therapies.
Cuproptosis, Lymphoma and Therapeutic Strategies
The role of cuproptosis has also been specifically investigated in the context of hematological malignancies, particularly lymphomas like Diffuse Large B-Cell Lymphoma (DLBCL) and related conditions such as Multiple Myeloma (MM),[44-50] acute lymphoblastic leukemia,[51] and myeloid neoplasms,[52-66] as documented in the reviewed articles. These studies explore the pathway's relevance for prognosis, its mechanistic involvement in lymphomagenesis, and its potential as a therapeutic target.Similar to the findings in solid tumors, several studies focused on the prognostic significance of cuproptosis-related molecular signatures in DLBCL. Researchers have developed and validated prognostic models based on the expression levels of specific cuproptosis-associated genes[67-68] or cuproptosis-related lncRNAs. These models aim to improve risk stratification for DLBCL patients beyond traditional clinical parameters. Further refining this approach, one study developed a combined prognostic model incorporating markers of both cuproptosis and immunogenic cell death, suggesting potential interplay between these processes in determining DLBCL outcomes.[69] The consistent ability of these signatures to predict prognosis underscores the intrinsic involvement of the cuproptosis pathway in the pathobiology of DLBCL.
Mechanistic investigations have begun to shed light on how cuproptosis pathways might directly influence lymphoma cell behavior. A key finding reported by Xie et al. implicates the cuproptosis-related gene ATOX1, which encodes a copper chaperone protein, in promoting DLBCL proliferation.[17] This study suggested that ATOX1 achieves this by modulating intracellular copper transport and potentially influencing downstream signaling pathways like MAPK signaling.[17] This provides a direct link between the cellular machinery regulating copper homeostasis, which is central to cuproptosis, and the control of lymphoma cell growth. The specific mechanisms and therapeutic potential of cuproptosis in lymphoma have also been the subject of focused reviews,[13] and broader narrative reviews on novel therapeutic approaches in DLBCL acknowledge the potential relevance of targeting tumor metabolism, including copper pathways.[24]
Building on these prognostic and mechanistic insights, therapeutic strategies targeting cuproptosis are being explored for lymphomas and related B-cell malignancies. For instance, Wang et al. described the development of UiO-66 metal-organic framework (MOF)-based nano-sonosensitizers designed for ultrasound-activated immunotherapy against B-cell lymphoma.[70] While the precise contribution of cuproptosis needs further clarification, such approaches targeting cellular stress pathways, potentially including copper-induced stress, represent innovative therapeutic directions. In the context of Multiple Myeloma (MM), another B-cell malignancy, Wang and colleagues reported an intriguing finding related to drug resistance.[16] They found that the protein MUC20, whose expression was regulated by extrachromosomal circular DNA, could modulate cuproptosis sensitivity and thereby attenuate resistance to proteasome inhibitors, a standard class of drugs used in MM treatment.[16] This suggests that manipulating cuproptosis sensitivity could represent a novel strategy to overcome or circumvent acquired drug resistance in MM and potentially other lymphomas. All the abovementioned studies regarding the interplay between cuproptosis and lymphoproliferative disorders are summarized in Table 1.
Collectively, the reviewed literature indicates that cuproptosis is a relevant biological process in lymphoma. Molecular signatures related to this pathway hold prognostic value in DLBCL, specific pathway components like ATOX1 are implicated in regulating lymphoma cell proliferation, and targeting copper metabolism or inducing cuproptosis is emerging as a potential therapeutic avenue, including strategies aimed at tackling drug resistance in related malignancies.
Cuproptosis and Viral Infections
The intersection between cuproptosis and viral infections represents a relatively nascent but potentially significant area of investigation, with a few studies in the reviewed set providing initial insights into these interactions.Research related to the COVID-19 pandemic explored potential links between cuproptosis and the host response to SARS-CoV-2 infection. Luo et al. employed machine learning techniques to identify distinct molecular subtypes of COVID-19 based on the expression of cuproptosis-related genes and developed a novel predictive model for disease outcomes.[71] This suggests that alterations in cuproptosis pathways might correlate with disease severity or specific host response patterns during SARS-CoV-2 infection. Another study focused on patients with non-small cell lung cancer (NSCLC) who were co-infected with COVID-19.[49] Li and colleagues investigated the prognostic impact of cuproptosis-associated lncRNAs in this specific patient population, indicating a potential complex interplay between the underlying cancer, the viral infection, and the regulation of this particular cell death pathway.[37] These findings hint that cuproptosis might be involved in the systemic metabolic and inflammatory disturbances characteristic of severe COVID-19, or that the virus itself might modulate cellular copper handling or mitochondrial function.
Evidence for viral manipulation of host cell death pathways also comes from studies on other viruses. Cao et al. utilized transcriptomic analysis to investigate how Pseudorabies Virus (PRV) infection affects cell death regulation in neuroblastoma cells.[15] Their findings indicated that PRV infection leads to a suppression of host cell death pathways, likely as a viral strategy to promote its own replication and survival within the host cell.[15] While cuproptosis was not explicitly confirmed as one of the suppressed pathways in the provided summary, this study highlights the general principle that viruses can evolve mechanisms to counteract host cell death programs. Although direct studies linking cuproptosis and Epstein-Barr Virus (EBV) were not present in the reviewed literature, EBV is known to manipulate host cell metabolism, including mitochondrial functions, to support latent infection and B-cell transformation. Therefore, investigating potential intersections between EBV infection and cuproptosis pathways via shared mitochondrial metabolic pathways could be a relevant future direction.
In summary, the reviewed articles provide preliminary but intriguing evidence suggesting that cuproptosis may play a role in the context of viral infections, potentially influencing host responses (as suggested for COVID-19) or being targeted by viruses to evade host defenses (as suggested for PRV). Further research is clearly warranted to elucidate the specific mechanisms and functional significance of cuproptosis during various viral infections.
Discussion and Future Directions
This review underscores the rapid emergence of cuproptosis as a distinct and significant field within cell death research, possessing considerable relevance for oncology, hematology and potentially infectious diseases. Its unique mechanism, fundamentally linked to copper overload disrupting mitochondrial function through the aggregation of lipoylated proteins and subsequent proteotoxic stress,[13] sets it apart from apoptosis, necroptosis, and ferroptosis. This distinctiveness offers novel avenues for both understanding disease pathogenesis and developing targeted therapeutic interventions.A major theme emerging from synthesized literature is the profound connection between cuproptosis pathways and cancer biology. The consistent identification of prognostic signatures based on cuproptosis-related genes and lncRNAs across a wide array of solid tumors — including breast,[35] gastrointestinal,[27,28,29,36] lung,[21,22,30-32] neuroblastoma,[23,33] and pancreatic[26] — as well as in lymphoma,[34,68,69] strongly supports the fundamental role of copper metabolism and this specific cell death modality in tumor progression and clinical outcome. These molecular signatures hold considerable promise as biomarkers for improved patient stratification, prediction of treatment response (to immunotherapy, chemotherapy, or radiotherapy[31,36]), and potentially guiding personalized medicine approaches, although rigorous prospective validation remains a critical next step.
The therapeutic potential of deliberately inducing cuproptosis in cancer cells is arguably the most dynamic area highlighted in the reviewed literature. The strategies being explored are diverse, ranging from repurposing existing drugs like Disulfiram[22] and utilizing copper ionophores such as Elesclomol[38,39] to the rational design of sophisticated nanomedicine platforms.[16,23,25,38,40-43,68-70] These nanocarriers represent a significant advancement, offering potential solutions to challenges of systemic toxicity and enabling targeted delivery of copper or cuproptosis inducers to the tumor site, often incorporating stimuli-responsive release mechanisms. Furthermore, the emphasis on synergistic combinations — pairing cuproptosis induction with immunotherapy,[16,24,38,41] chemotherapy,[25,40] sonodynamic therapy,[16,41,43] or even co-triggering other RCD pathways like ferroptosis[23] — reflects a sophisticated approach aimed at maximizing anti-cancer efficacy and overcoming the inherent heterogeneity and adaptability of tumors. However, translating these promising preclinical findings into effective clinical therapies will require overcoming significant hurdles, including optimizing delivery efficiency, ensuring acceptable safety profiles, and developing strategies to counteract intrinsic or acquired resistance mechanisms, such as those mediated by HIF-1α under hypoxia[19] or protective proteins like metallothioneins.[14] Buccarelli et al.[71] explore the combination of elesclomol with temozolomide, which enhances cytotoxicity in vitro and reduces tumor growth in vivo, suggesting a promising therapeutic strategy for glioblastoma.
The nascent exploration of links between cuproptosis and viral infections[15,30,72] opens another intriguing research frontier. Understanding the bidirectional interactions — how viral infections might perturb cellular copper homeostasis and mitochondrial function to modulate cuproptosis sensitivity, and conversely, how cuproptosis might contribute to antiviral host defense or viral pathogenesis and associated inflammation — could yield novel insights into infectious disease mechanisms and potentially new therapeutic targets. The hypothesis regarding a potential link with EBV, based on its known manipulation of mitochondrial metabolism, warrants investigation.
Despite the remarkable progress documented in these articles, several key areas necessitate further investigation. Firstly, a deeper mechanistic understanding is required, particularly regarding the precise downstream execution events following mitochondrial proteotoxic stress and Fe-S cluster loss, as well as the full spectrum of upstream regulatory inputs and crosstalk with other cellular pathways like autophagy. Secondly, the prognostic and predictive biomarkers identified primarily through bioinformatic analyses require stringent validation in large, independent, and prospectively collected patient cohorts, coupled with functional studies to confirm their mechanistic roles. Thirdly, the path to clinical translation for cuproptosis-inducing therapies requires careful navigation of challenges related to pharmacokinetics, biodistribution, long-term toxicity, and the development of robust strategies to monitor treatment response and manage resistance. Fourthly, recognizing the importance of context, future studies should delve deeper into how the role and regulation of cuproptosis vary depending on the specific cancer type, its genetic background, and the complexities of the tumor microenvironment. Finally, the potential involvement of cuproptosis in diseases beyond cancer and viral infections, such as neurodegenerative disorders, cardiovascular conditions (as hinted by the doxorubicin cardiomyopathy study[14]), or metabolic diseases where copper dyshomeostasis is implicated, remains largely uncharted territory ripe for exploration.
Conclusions
In conclusion, this review, based on the analysis of the available research articles, portrays cuproptosis as a distinct, copper-dependent mode of regulated cell death centered on mitochondrial dysfunction and proteotoxicity. The synthesized evidence strongly highlights its relevance as a prognostic factor in diverse malignancies, including numerous solid tumors and lymphomas, and underscores its potential as a novel therapeutic target in oncology. The development of innovative therapeutic strategies, particularly those employing nanomedicine for targeted delivery and synergistic combinations with other modalities like immunotherapy, reflects significant translational interest. Furthermore, emerging findings connecting cuproptosis to viral infections suggest broader physiological and pathological roles that warrant further investigation. Continued research dedicated to unraveling the intricate molecular mechanisms, validating clinical biomarkers, refining therapeutic approaches, and exploring the broader biological significance of cuproptosis holds substantial promise for advancing our fundamental understanding of cell death and potentially yielding new therapeutic paradigms for cancer and other human diseases.Author Contributions
P.T., L.M.L. and M.M. were the principal authors and main contributors to writing the manuscript. M.B., W.G., V.F., and C.P. reviewed the literature. L.M.L., V.Z. and A.B. have read and revised the manuscript. G.T., A.I and G.F. revised the English language. All authors have read and approved the final manuscript.Acknowledgments
We thank Dr. Rosa Scarfì for her technical support.References
- Xue Y, Jiang X, Wang J, Zong Y, Yuan Z, Miao S, Mao X. Effect of regulatory cell death on the occurrence and development of head and neck squamous cell carcinoma. Biomark Res. 2023 Jan 5;11(1):2. doi: 10.1186/s40364-022-00433-w. https://doi.org/10.1186/s40364-022-00433-w PMid:36600313 PMCid:PMC9814270
- Chen
R, Zou J, Kang R, Tang D. The Redox Protein High-Mobility Group Box 1
in Cell Death and Cancer. Antioxid Redox Signal. 2023
Sep;39(7-9):569-590. doi: 10.1089/ars.2023.0236. Epub 2023 Mar 30. https://doi.org/10.1089/ars.2023.0236 PMid:36999916
- Chen,
L., Min, J., & Wang, F. (2022). Copper homeostasis and cuproptosis
in health and disease. Signal Transduction and Targeted Therapy, 7,
378. https://doi.org/10.1038/s41392-022-01229-y PMid:36414625 PMCid:PMC9681860
- Ramos,
D., Mar, D., Ishida, M., Vargas, R., Gaite, M., Montgomery, A., &
Linder, M. C. (2016). Mechanism of Copper Uptake from Blood Plasma
Ceruloplasmin by Mammalian Cells. PloS One, 11(3), e0149516. https://doi.org/10.1371/journal.pone.0149516 PMid:26934375 PMCid:PMC4774968
- Festa, R. A., & Thiele, D. J. (2011). Copper: an essential metal in biology. Current Biology : CB, 21(21), R877-R883. https://doi.org/10.1016/j.cub.2011.09.040 PMid:22075424 PMCid:PMC3718004
- Svetlana
Lutsenko, Copper trafficking to the secretory pathway, Metallomics,
Volume 8, Issue 9, September 2016, Pages 840-852. https://doi.org/10.1039/C6MT00176A PMid:27603756 PMCid:PMC5548098
- Liang,
Z. D., Tsai, W. B., Lee, M. Y., Savaraj, N., & Kuo, M. T. (2012).
Specificity protein 1 (sp1) oscillation is involved in copper
homeostasis maintenance by regulating human high-affinity copper
transporter 1 expression. Molecular Pharmacology, 81(3), 455-464. https://doi.org/10.1124/mol.111.076422 PMid:22172574 PMCid:PMC3286298
- Boyd,
S. D., Ullrich, M. S., Skopp, A., & Winkler, D. D. (2020). Copper
Sources for Sod1 Activation. Antioxidants (Basel, Switzerland), 9(6),
500. https://doi.org/10.3390/antiox9060500 PMid:32517371 PMCid:PMC7346115
- La
Fontaine, S., Ackland, M. L., & Mercer, J. F. (2010). Mammalian
copper-transporting P-type ATPases, ATP7A and ATP7B: emerging roles.
The International Journal of Biochemistry & Cell Biology, 42(2),
206-209. https://doi.org/10.1016/j.biocel.2009.11.007 PMid:19922814 PMCid:PMC2846448
- Prohaska
J. R. (2008). Role of copper transporters in copper homeostasis. The
American Journal of Clinical Nutrition, 88(3), 826S-9S. https://doi.org/10.1093/ajcn/88.3.826S PMid:18779302 PMCid:PMC2799992
- Lutsenko,
S., Bhattacharjee, A., & Hubbard, A. L. (2010). Copper handling
machinery of the brain. Metallomics: Integrated Biometal Science,
2(9), 596-608. https://doi.org/10.1039/c0mt00006j PMid:21072351
- Maung,
M. T., Carlson, A., Olea-Flores, M., Elkhadragy, L., Schachtschneider,
K. M., Navarro-Tito, N., & Padilla-Benavides, T. (2021). The
molecular and cellular basis of copper dysregulation and its
relationship with human pathologies. FASEB journal : official
publication of the Federation of American Societies for Experimental
Biology, 35(9), e21810. https://doi.org/10.1096/fj.202100273RR PMid:34390520
- Wang
Y, Yin F, Jin Q, Liu C, Qi Z, Chen D, Luo Y. Cuproptosis: Mechanism and
Application in Lymphoma. Curr Cancer Drug Targets. 2024 Jul 11. doi:
10.2174/0115680096296742240614100116. Epub ahead of print. https://doi.org/10.2174/0115680096296742240614100116 PMID: 38994618
- Liu
Y, Shen M, Zhu S, Du Z, Lin L, Ma J, Reiter RJ, Ashrafizadeh M, Li G,
Zou R, Su H, Ren J, Lu Q. Metallothionein rescues doxorubicin
cardiomyopathy via mitigation of cuproptosis. Life Sci. 2025 Feb
15;363:123379. doi: 10.1016/j.lfs.2025.123379. Epub 2025 Jan 8. https://doi.org/10.1016/j.lfs.2025.123379 PMid:39793852
- Cao
S, Zhang L, Zhou M, Zhu S. Transcriptomic insights into pseudorabies
virus suppressed cell death pathways in neuroblastoma cells. Front
Microbiol. 2024 Sep 19;15:1430396. doi:
10.3389/fmicb.2024.1430396. https://doi.org/10.3389/fmicb.2024.1430396 PMid:39364165 PMCid:PMC11447949
- Wang
X, Shi Y, Shi H, Liu X, Liao A, Liu Z, Orlowski RZ, Zhang R, Wang H.
MUC20 regulated by extrachromosomal circular DNA attenuates proteasome
inhibitor resistance of multiple myeloma by modulating cuproptosis. J
Exp Clin Cancer Res. 2024 Mar 5;43(1):68. doi:
10.1186/s13046-024-02972-6. https://doi.org/10.1186/s13046-024-02972-6 PMid:38439082 PMCid:PMC10913264
- Xie
J, Shao Z, Li C, Zeng C, Xu B. Cuproptosis-related gene ATOX1 promotes
MAPK signaling and diffuse large B-cell lymphoma proliferation via
modulating copper transport. Biomol Biomed. 2024 Dec 11;25(1):16-28.
doi: 10.17305/bb.2024.10536. https://doi.org/10.17305/bb.2024.10536 PMid:39036924 PMCid:PMC11647247
- Sun
L, Shao W, Lin Z, Lin J, Zhao F, Yu J. Single-cell RNA sequencing
explored potential therapeutic targets by revealing the tumor
microenvironment of neuroblastoma and its expression in cell death.
Discov Oncol. 2024 Sep 5;15(1):409. doi: 10.1007/s12672-024-01286-5. https://doi.org/10.1007/s12672-024-01286-5 PMid:39235657 PMCid:PMC11377405
- Yang
Z, Su W, Wei X, Pan Y, Xing M, Niu L, Feng B, Kong W, Ren X, Huang F,
Zhou J, Zhao W, Qiu Y, Liao T, Chen Q, Qu S, Wang Y, Guan Q, Li D, Zen
K, Chen Y, Qin C, Wang Y, Zhou X, Xiang J, Yao B. Hypoxia inducible
factor-1α drives cancer resistance to cuproptosis. Cancer Cell. 2025
Mar 6:S1535-6108(25)00067-4. doi: 10.1016/j.ccell.2025.02.015. Epub
ahead of print. https://doi.org/10.1016/j.ccell.2025.02.015 PMid:40054467
- Chen
TH, Lin SH, Lee MY, Wang HC, Tsai KF, Chou CK. Mitochondrial
alterations and signatures in hepatocellular carcinoma. Cancer
Metastasis Rev. 2025 Feb 18;44(1):34. doi:
10.1007/s10555-025-10251-9. https://doi.org/10.1007/s10555-025-10251-9 PMid:39966277 PMCid:PMC11836208
- Lyu
G, Dai L, Deng X, Liu X, Guo Y, Zhang Y, Wang X, Huang Y, Wu S, Guo JC,
Liu Y. Integrative Analysis of Cuproptosis-Related Mitochondrial
Depolarisation Genes for Prognostic Prediction in Non-Small Cell Lung
Cancer. J Cell Mol Med. 2025 Feb;29(4):e70438. doi: 10.1111/jcmm.70438.
https://doi.org/10.1111/jcmm.70438 PMid:40008552 PMCid:PMC11862892
- Gan
Y, Liu T, Feng W, Wang L, Li LI, Ning Y. Drug repositioning of
disulfiram induces endometrioid epithelial ovarian cancer cell death
via the both apoptosis and cuproptosis pathways. Oncol Res. 2023 May
24;31(3):333-343. doi: 10.32604/or.2023.028694. https://doi.org/10.32604/or.2023.028694 PMid:37305383 PMCid:PMC10229305
- Chen
Y, Liu J, Jia Y, Yang J, Jin Y, Liu Y, Zhong B, Zhao Q. Cell death
pathway regulation by fatty acid metabolism-related genes in
neuroblastoma: a multi-omics analysis identifying CHD5 as a novel
biomarker. Discov Oncol. 2025 Mar 23;16(1):377. doi:
10.1007/s12672-025-02088-z. https://doi.org/10.1007/s12672-025-02088-z PMid:40121577 PMCid:PMC11930905
- Masnikosa
R, Cvetković Z, Pirić D. Tumor Biology Hides Novel Therapeutic
Approaches to Diffuse Large B-Cell Lymphoma: A Narrative Review. Int J
Mol Sci. 2024 Oct 23;25(21):11384. doi: 10.3390/ijms252111384. https://doi.org/10.3390/ijms252111384 PMid:39518937 PMCid:PMC11545713
- Luo
Y, Wang Y, Liu B, Liu Y, Zhang W, Chen S, Rong X, Xu L, Du Q, Liu J, Xu
J, Ran H, Wang Z, Guo D. A
<i>"</i>CPApoptosis<i>"</i> nano-actuator
switches immune-off solid tumors to immune-on for fueling T-cell- based
immunotherapy. Theranostics. 2025 Mar 3;15(9):3797-3820. doi:
10.7150/thno.105867. https://doi.org/10.7150/thno.105867 PMid:40213664 PMCid:PMC11980655
- Liu
H. Pan-cancer profiles of the cuproptosis gene set. Am J Cancer Res.
2022 Aug 15;12(8):4074-4081. PMID: 36119826; PMCID: PMC9442004.
- Tang
J, Yang J, Yin LK. Prognostic value of disulfidptosis-associated genes
in gastric cancer: a comprehensive analysis. Front Oncol. 2025 Mar
4;15:1512394. doi: 10.3389/fonc.2025.1512394. https://doi.org/10.3389/fonc.2025.1512394 PMid:40104507 PMCid:PMC11913695
- Yan
JN, Guo LH, Zhu DP, Ye GL, Shao YF, Zhou HX. Clinical significance and
potential application of cuproptosis-related genes in gastric cancer.
World J Gastrointest Oncol. 2023 Jul 15;15(7):1200-1214. doi:
10.4251/wjgo.v15.i7.1200. https://doi.org/10.4251/wjgo.v15.i7.1200 PMid:37546553 PMCid:PMC10401470
- Liu
XS, Liu C, Zeng J, Zeng DB, Chen YJ, Tan F, Gao Y, Liu XY, Zhang Y,
Zhang YH, Pei ZJ. Nucleophosmin 1 is a prognostic marker of
gastrointestinal cancer and is associated with m6A and cuproptosis.
Front Pharmacol. 2022 Sep 14;13:1010879. doi:
10.3389/fphar.2022.1010879. https://doi.org/10.3389/fphar.2022.1010879 PMid:36188614 PMCid:PMC9515486
- Li
J, Wang N, Mao G, Wang J, Xiang M, Zhang H, Zeng D, Ma H, Jiang J.
Cuproptosis-associated lncRNA impact prognosis in patients with
non-small cell lung cancer co-infected with COVID-19. J Cell Mol Med.
2024 Sep;28(17):e70059. doi: 10.1111/jcmm.70059. https://doi.org/10.1111/jcmm.70059 PMid:39228012 PMCid:PMC11371660
- Xu
Q, Liu T, Wang J. Radiosensitization-Related Cuproptosis LncRNA
Signature in Non-Small Cell Lung Cancer. Genes (Basel). 2022 Nov
9;13(11):2080. doi: 10.3390/genes13112080. https://doi.org/10.3390/genes13112080 PMid:36360316 PMCid:PMC9690519
- Sun
X, Li Z, Meng F, Huang X, Wang J, Song J, Sun L, Zhang P. Cuproptosis
associated genes affect prognosis and tumor microenvironment
infiltration characterization in lung adenocarcinoma. Am J Cancer Res.
2022 Oct 15;12(10):4545-4565. PMID: 36381320; PMCID: PMC9641400.
- Tian
XM, Xiang B, Yu YH, Li Q, Zhang ZX, Zhanghuang C, Jin LM, Wang JK, Mi
T, Chen ML, Liu F, Wei GH. A novel cuproptosis-related subtypes and
gene signature associates with immunophenotype and predicts prognosis
accurately in neuroblastoma. Front Immunol. 2022 Sep 23;13:999849. doi:
10.3389/fimmu.2022.999849. https://doi.org/10.3389/fimmu.2022.999849 PMid:36211401 PMCid:PMC9540510
- Wang
N, Shi S, Li M, Yu X, Ma G. Development and validation of a combined
cuproptosis and immunogenic cell death prognostic model for diffuse
large B-cell lymphoma. Aging (Albany, NY). 2024 Jan 26;16(2):1218-1236.
doi: 10.18632/aging.205399. Epub 2024 Jan 26. https://doi.org/10.18632/aging.205399 PMid:38284893 PMCid:PMC10866411
- Li
T, Wang D, Meng M, Yang Z, Luo Z, Li Z, Li F, Liu C, Hao K, Pang X,
Tian H, Chen X. Copper-Coordinated Covalent Organic Framework Produced
a Robust Fenton-Like Effect Inducing Immunogenic Cell Death of Tumors.
Macromol Rapid Commun. 2023 Jun;44(11):e2200929. doi:
10.1002/marc.202200929. Epub 2023 Mar 12. https://doi.org/10.1002/marc.202200929 PMid:36840703
- Chen
K, Zhou A, Zhou X, Liu Y, Xu Y, Ning X. An Intelligent Cell-Derived
Nanorobot Bridges Synergistic Crosstalk Between Sonodynamic Therapy and
Cuproptosis to Promote Cancer Treatment. Nano Lett. 2023 Apr
12;23(7):3038-3047. doi: 10.1021/acs.nanolett.3c00434. Epub 2023 Mar
23. https://doi.org/10.1021/acs.nanolett.3c00434 PMid:36951267
- Li
C, Zhang K, Gong Y, Wu Q, Zhang Y, Dong Y, Li D, Wang Z. Based on
cuproptosis-related lncRNAs, a novel prognostic signature for colon
adenocarcinoma prognosis, immunotherapy, and chemotherapy response.
Front Pharmacol. 2023 Jun 12;14:1200054. doi:
10.3389/fphar.2023.1200054. https://doi.org/10.3389/fphar.2023.1200054 PMid:37377924 PMCid:PMC10291194
- Cao
C, Wang T, Luo Y, Zhang Y, Dai YY, Shen Y. Comprehensive analysis of
cuproptosis-associated LncRNAs predictive value and related CeRNA
network in acute myeloid leukemia. Heliyon. 2023 Nov 25;9(12):e22532.
doi: 10.1016/j.heliyon.2023.e22532. https://doi.org/10.1016/j.heliyon.2023.e22532 PMid:38058427 PMCid:PMC10696213
- Tarin
M, Babaie M, Eshghi H, Matin MM, Saljooghi AS. Elesclomol, a
copper-transporting therapeutic agent targeting mitochondria: from
discovery to its novel applications. J Transl Med. 2023 Oct
20;21(1):745. doi: 10.1186/s12967-023-04533-5. https://doi.org/10.1186/s12967-023-04533-5 PMid:37864163 PMCid:PMC10589935
- Lu
X, Chen X, Lin C, Yi Y, Zhao S, Zhu B, Deng W, Wang X, Xie Z, Rao S, Ni
Z, You T, Li L, Huang Y, Xue X, Yu Y, Sun W, Shen X. Elesclomol Loaded
Copper Oxide Nanoplatform Triggers Cuproptosis to Enhance Antitumor
Immunotherapy. Adv Sci (Weinh). 2024 May;11(18):e2309984. doi:
10.1002/advs.202309984. Epub 2024 Mar 2. https://doi.org/10.1002/advs.202309984 PMid:38430531 PMCid:PMC11095170
- Wang
S, Liu Y, Su M, Wang Y, Wang W, Wang W, Yang Q, Zhang Z, Hong X, Sun Z,
Xiao Y. Near-Infrared Activatable Copper Nanoplatforms Synergize with
the 5-Azacytidine Prodrug to Potentiate Cuproptosis. Angew Chem Int Ed
Engl. 2024 Dec 20;63(52):e202411609. doi: 10.1002/anie.202411609. Epub
2024 Nov 9. https://doi.org/10.1002/anie.202411609 PMid:39400411
- Zhang
N, Yu X, Sun H, Zhao Y, Wu J, Liu G. A prognostic and immunotherapy
effectiveness model for pancreatic adenocarcinoma based on
cuproptosis-related lncRNAs signature. Medicine (Baltimore). 2023 Oct
20;102(42):e35167. doi: 10.1097/MD.0000000000035167. https://doi.org/10.1097/MD.0000000000035167 PMid:37861553 PMCid:PMC10589590
- Liu
J, Chen Z, Deng L, Yao C, Zhou Z, Zhou C, Bin Y, Liu M, Wang L, Wang L,
Wang Z. Metal-phenolic networks specifically eliminate hypoxic tumors
by instigating oxidative and proteotoxic stresses. Bioact Mater. 2025
Feb 12;47:361-377. doi: 10.1016/j.bioactmat.2025.01.022. https://doi.org/10.1016/j.bioactmat.2025.01.022 PMid:40026824 PMCid:PMC11870026
- Zhang,
B., Wang, Q., Zhang, T., Zheng, Z., Lin, Z., Zhou, S., Zheng, D., Chen,
Z., Zheng, S., Zhang, Y., Lin, X., Dong, R., Chen, J., Qian, H., Hu,
X., Zhuang, Y., Zhang, Q., Jin, Z., Jiang, S., & Ma, Y. (2023).
Identification and validation of a novel cuproptosis-related gene
signature in multiple myeloma. Frontiers in Cell and Developmental Biology, 11, 1159355. https://doi.org/10.3389/fcell.2023.1159355 PMid:37152283 PMCid:PMC10157051
- Wang,
H., Zhang, G., Dong, L., Chen, L., Liang, L., Ge, L., Gai, D., &
Shen, X. (2023). Identification and study of cuproptosis-related genes
in prognostic model of multiple myeloma. Hematology (Amsterdam,
Netherlands), 28(1), 2249217. https://doi.org/10.1080/16078454.2023.2249217 PMid:37610069
- Liu,
H., Chan, S., Li, M., & Chen, S. (2024). Cuproptosis-Related Gene
Signature Contributes to Prognostic Prediction and Immunosuppression in
Multiple Myeloma. Molecular Biotechnology, 66(3), 475-488. https://doi.org/10.1007/s12033-023-00770-7 PMid:37213025
- Chen,
Y., Tang, J., Chen, L., & Chen, J. (2023). Novel
cuproptosis-related lncRNAs can predict the prognosis of patients with
multiple myeloma. Translational Cancer Research, 12(11), 3074-3087. https://doi.org/10.21037/tcr-23-960 PMid:38130312 PMCid:PMC10731335
- Chen,
Y., Liu, J., & Zhu, Y. (2025). MAMDC2-AS1 Induces Cuproptosis in
Relapsed and Refractory Multiple Myeloma. Cancer Reports (Hoboken,
N.J.), 8(5), e70216. https://doi.org/10.1002/cnr2.70216 PMid:40344606 PMCid:PMC12062513
- Li,
T., Yao, L., Hua, Y., & Wu, Q. (2023). Comprehensive analysis of
prognosis of cuproptosis-related oxidative stress genes in multiple
myeloma. Frontiers in Genetics, 14, 1100170. https://doi.org/10.3389/fgene.2023.1100170 PMid:37065484 PMCid:PMC10102368
- Gao,
X. H., Yuan, J., Zhang, X. X., Wang, R. C., Yang, J., Li, Y., & Li,
J. (2025). Exploration of the Prognostic Markers of Multiple Myeloma
Based on Cuproptosis-Related Genes. Cancer Reports (Hoboken, N.J.),
8(3), e70151. https://doi.org/10.1002/cnr2.70151 PMid:40042106 PMCid:PMC11880913
- Zhang,
B., Zhu, S., Zheng, D., Zhang, X., Xie, W., Zhou, S., Zheng, S., Wang,
Q., Lin, Z., Zheng, Z., Chen, Z., Lan, E., Cui, L., Ying, H., Zhang,
Y., Lin, X., Zhuang, Q., Qian, H., Hu, X., Zhuang, Y., … Ma, Y. (2025).
Development of a cuproptosis-related prognostic signature to reveal
heterogeneity of the immune microenvironment and drug sensitivity in
acute lymphoblastic leukemia. European Journal of Medical Research,
30(1), 435. https://doi.org/10.1186/s40001-025-02572-w PMid:40450339 PMCid:PMC12125805
- Luo,
D., Liu, S., Luo, J., Chen, H., He, Z., Gao, Z., Wen, Z., Liu, X.,
& Xu, N. (2023). Characterization of cuproptosis identified immune
microenvironment and prognosis in acute myeloid leukemia. Clinical
& translational oncology: official publication of the Federation of
Spanish Oncology Societies and of the National Cancer Institute of
Mexico, 25(8), 2393-2407. https://doi.org/10.1007/s12094-023-03118-4 PMid:36826709
- Li,
Y., & Kan, X. (2024). Cuproptosis-Related Genes MTF1 and LIPT1 as
Novel Prognostic Biomarker in Acute Myeloid Leukemia. Biochemical Genetics, 62(2), 1136-1159. https://doi.org/10.1007/s10528-023-10473-y PMid:37561332
- Li,
P., Li, J., Wen, F., Cao, Y., Luo, Z., Zuo, J., Wu, F., Li, Z., Li, W.,
& Wang, F. (2022). A novel cuproptosis-related LncRNA signature:
Prognostic and therapeutic value for acute myeloid leukemia. Frontiers
in Oncology, 12, 966920. https://doi.org/10.3389/fonc.2022.966920 PMid:36276132 PMCid:PMC9585311
- Tao,
Y., Ren, J., Xue, T., Wang, Y., Xu, H., Zhang, H., & Lu, J. (2023).
Determination of Cuproptosis-related Subtypes, Development of a
Prognostic Model, and Characterization of Tumor Microenvironment
Infiltration in Acute Myeloid Leukemia. Anticancer Research, 43(9),
3943-3960. https://doi.org/10.21873/anticanres.16582 PMid:37648328
- Wu,
M., Li, A., Zhang, T., Ding, W., Wei, Y., Wan, C., Ke, B., Cheng, H.,
Jin, C., & Kong, C. (2024). The novel prognostic analysis of AML
based on ferroptosis and cuproptosis related genes. Journal of Trace
Elements in Medicine and Biology: organ of the Society for Minerals
and Trace Elements (GMS), 86, 127517. https://doi.org/10.1016/j.jtemb.2024.127517 PMid:39270538
- Qin,
Y., Pu, X., Hu, D. et al. Machine learning-based biomarker screening
for acute myeloid leukemia prognosis and therapy from diverse
cell-death patterns. Sci Rep 14, 17874 (2024). https://doi.org/10.1038/s41598-024-68755-3 PMid:39090256 PMCid:PMC11294352
- Li,
H., Li, Y., Yu, Y., Ren, X., Yang, C., Jin, W., Li, K., Zhou, Y., Wu,
C., Shen, Y., Hu, W., Liu, Y., Yu, L., Tong, X., Du, J., & Wang, Y.
(2024). GSH exhaustion via inhibition of xCT-GSH-GPX4 pathway
synergistically enhanced DSF/Cu-induced cuproptosis in myelodysplastic
syndromes. Free Radical Biology & Medicine, 222, 130-148. https://doi.org/10.1016/j.freeradbiomed.2024.06.006 PMid:38866192
- Chai,
Z., Yuan, Z., & Chen, Y. (2025). Stem cell status and prognostic
applications of cuproptosis-associated lncRNAs in acute myeloid
leukemia. Frontiers in Cell and Developmental Biology, 12, 1549294. https://doi.org/10.3389/fcell.2024.1549294 PMid:39877157 PMCid:PMC11772438
- Song,
Y., Yang, Z., Gao, N., & Zhang, B. (2024). MICAL1 promotes the
proliferation in acute myeloid leukemia and is associated with clinical
prognosis and immune infiltration. Discover Oncology, 15(1), 279. https://doi.org/10.1007/s12672-024-01150-6 PMid:38995414 PMCid:PMC11245461
- Wang,
X., Sun, H., Dong, Y., Huang, J., Bai, L., Tang, Z., Liu, S., &
Chen, S. (2024). Development and validation of a cuproptosis-related
prognostic model for acute myeloid leukemia patients using machine
learning with stacking. Scientific Reports, 14(1), 2802. https://doi.org/10.1038/s41598-024-53306-7 PMid:38307903 PMCid:PMC10837443
- Abulimiti,
M., Jia, Z. Y., Wu, Y., Yu, J., Gong, Y. H., Guan, N., Xiong, D. Q.,
Ding, N., Uddin, N., & Wang, J. (2024). Exploring and clinical
validation of prognostic significance and therapeutic implications of
copper homeostasis-related gene dysregulation in acute myeloid
leukemia. Annals of Hematology, 103(8), 2797-2826. https://doi.org/10.1007/s00277-024-05841-6 PMid:38879648
- Cao,
J., Xie, M., Sun, K., Zhao, Y., Zheng, J., Wang, Y., Zheng, Y., Liu,
S., & Yu, U. (2024). Development of a prognostic model
incorporating a cuproptosis-related signature and CNN3 as a predictor
in childhood acute myelocytic leukemia. Frontiers in Oncology, 14,
1494777. https://doi.org/10.3389/fonc.2024.1494777 PMid:39555457 PMCid:PMC11564170
- Zhang,
T., Liao, D., & Hu, Y. (2023). Cuproptosis-related lncRNAs forecast
the prognosis of acute myeloid leukemia. Translational Cancer Research,
12(5), 1175-1195. https://doi.org/10.21037/tcr-22-2526 PMid:37304546 PMCid:PMC10248575
- Zhu,
Y., He, J., Li, Z., & Yang, W. (2023). Cuproptosis-related lncRNA
signature for prognostic prediction in patients with acute myeloid
leukemia. BMC Bioinformatics, 24(1), 37. https://doi.org/10.1186/s12859-023-05148-9 PMid:36737692 PMCid:PMC9896718
- Moison,
C., Gracias, D., Schmitt, J., Girard, S., Spinella, J. F., Fortier, S.,
Boivin, I., Mendoza-Sanchez, R., Thavonekham, B., MacRae, T., Mayotte,
N., Bonneil, E., Wittman, M., Carmichael, J., Ruel, R., Thibault, P.,
Hébert, J., Marinier, A., & Sauvageau, G. (2024). SF3B1 mutations
provide genetic vulnerability to copper ionophores in human acute
myeloid leukemia. Science Advances, 10(12), eadl4018. https://doi.org/10.1126/sciadv.adl4018 PMid:38517966 PMCid:PMC10959413
- Chen,
W., Jiang, Y., Zeng, J. et al. FDX1 promotes elesclomol-induced
PANoptosis in diffuse large B-cell lymphoma via activating IRF3/IFN-β
signaling. Oncogene (2025). https://doi.org/10.1038/s41388-025-03366-4
- Bai
X, Lu F, Li S, Zhao Z, Wang N, Zhao Y, Ma G, Zhang F, Su X, Wang D, Ye
J, Li P, Ji C. Cuproptosis-related lncRNA signature as a prognostic
tool and therapeutic target in diffuse large B cell lymphoma. Sci Rep.
2024 Jun 5;14(1):12926. doi: 10.1038/s41598-024-63433-w. PMID:
38839842; PMCID: PMC11153514.
- Zhang
B, Zhang T, Zheng Z, Lin Z, Wang Q, Zheng D, Chen Z, Ma Y. Development
and validation of a cuproptosis-associated prognostic model for diffuse
large B-cell lymphoma. Front Oncol. 2023 Jan 12;12:1020566. doi:
10.3389/fonc.2022.1020566. https://doi.org/10.3389/fonc.2022.1020566 PMid:36713586 PMCid:PMC9877310
- Wang
Z, Han M, Wang Y, Wang N, Yang Y, Shao B, Miao Q, Shi Z, Yan F, Feng S.
UiO-66 MOFs-Based "Epi-Nano-Sonosensitizer" for Ultrasound-Driven
Cascade Immunotherapy against B-Cell Lymphoma. ACS Nano. 2025 Feb
18;19(6):6282-6298. doi: 10.1021/acsnano.4c15761. Epub 2025 Feb 7. https://doi.org/10.1021/acsnano.4c15761 PMid:39920081
- Buccarelli,
M., D'Alessandris, Q. G., Matarrese, P., Mollinari, C., Signore, M.,
Cappannini, A., Martini, M., D'Aliberti, P., De Luca, G., Pedini, F.,
Boe, A., Biffoni, M., Pallini, R., & Ricci-Vitiani, L. (2021).
Elesclomol-induced increase of mitochondrial reactive oxygen species
impairs glioblastoma stem-like cell survival and tumor growth. Journal
of experimental & clinical cancer research : CR, 40(1), 228. https://doi.org/10.1186/s13046-021-02031-4 PMid:34253243 PMCid:PMC8273992
- Luo H, Yan J, Zhang D, Zhou X. Identification of cuproptosis-related molecular subtypes and a novel predictive model of COVID-19 based on machine learning. Front Immunol. 2023 Jul 17;14:1152223. doi: 10.3389/fimmu.2023.1152223. https://doi.org/10.3389/fimmu.2023.1152223 PMid:37533853 PMCid:PMC10393044