Comorbidity assessment is essential for selecting transplant candidates and tailoring chemotherapy intensity. While the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) includes pre-transplant inflammatory bowel disease, it overlooks other GI vulnerabilities like surgical resections or malabsorption syndromes.[2]
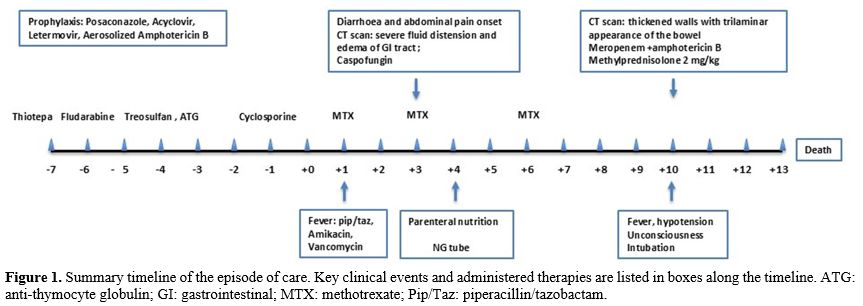
We present a clinical case that underscores how patients considered clinically “fit” — with no history of bowel disease or prior treatment — can still develop life-threatening GI toxicity, invasive infections, and fatal outcomes, emphasizing the need for more refined pre-transplant intestinal assessments (Figure 1).
A 69-year-old male was diagnosed with myelodysplastic/myeloproliferative neoplasm showing features of chronic myelomonocytic leukemia with fibrosis (CMML-F) in August 2023. Cytogenetics were normal, and next-generation sequencing identified mutations in ASXL1, JAK2, TET2, and U2AF1. WT1 was overexpressed. The bone marrow showed 8% blasts (3% inflow), the R-IPSS score was low, CPSS-mol intermediate-2, and CPSS high.
Aside from a remote sinusitis episode, his medical history was unremarkable. A retired excavator operator, he had no history of GI disease, and his HCT-CI score was 0. He was transfusion-dependent and receiving iron chelation therapy. A geriatric assessment deemed him fit, and pre-transplant imaging showed no organ damage.
Given the severity of the disease and the preservation of fitness, we intensified the conditioning regimen by adding thiotepa to treosulfan and fludarabine.[3]
In February 2024, he underwent conditioning: thiotepa 5 mg/kg BID (day -7), treosulfan 12,000 mg/m² (days -4 to -2), and fludarabine 30 mg/m² (days -6 to -2). GVHD prophylaxis included cyclosporine, ATG, and methotrexate. Antiviral and antifungal prophylaxis was administered; no antibacterial prophylaxis was used per protocol.
On day +1, he developed fever, elevated CRP (276 mg/L), procalcitonin (12.8 ng/mL), and lactate (2 mmol/L). Piperacillin-tazobactam and amikacin were started, followed by vancomycin after Streptococcus salivarius was isolated from central line blood cultures.
By day +3, he developed abdominal pain and diarrhea. Ultrasound showed diffuse intestinal distension, wall thickening, and fluid accumulation. Liver findings ruled out sinusoidal obstruction syndrome. A CT scan on day +4 revealed severe distension from the esophagus to the right colon and wall thickening of the left colon. A diagnosis of functional ileus was made, leading to nasogastric tube insertion and parenteral nutrition. Caspofungin was started empirically; rotavirus was weakly positive in stool cultures.
A repeat CT scan on day +10 showed trilaminar wall thickening in the colon and ileal loops, suggesting submucosal edema. Meropenem replaced piperacillin-tazobactam, and amphotericin B replaced caspofungin. Methylprednisolone 2 mg/kg was initiated to rule out hyperacute GVHD, with no benefit.
On day +13, he became unconscious and was intubated in the ICU. Imaging showed progressive bowel edema, ascites, splenic and renal infarcts, and pleural effusions. BAL and blood cultures later revealed Scedosporium prolificans and Apium complex. He died the next day of multiorgan failure. An autopsy showed widespread invasive mycosis with fungal elements and ischemic necrosis in multiple organs, including the GI tract.
Discussion
This case underscores the need to improve risk assessment for GI toxicity in allo-HSCT. Although disease features and apparent fitness justified intensified conditioning, it likely contributed to fatal mucosal injury.Current tools inadequately evaluate “gut fitness.” Evidence points to multiple avenues for better assessment. A systematic review by Wardill et al. identified dosimetric parameters, genetic variations in drug metabolism and immune response, and patient-specific factors as predictors of mucositis.[4]
Biomarkers such as serum citrulline, inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6), C-reactive protein, intestinal fatty acid-binding protein, fecal calprotectin, and calgranulin (S100A12) offer promising insights into mucosal barrier integrity.[5] Pontoppidan et al. showed lactulose-mannitol tests and serum citrulline correlated with pro-inflammatory microRNA profiles during early post-transplant periods.[6]
Nutritional and metabolic factors are also key. The EBMT Complications Working Party highlighted obesity and diabetes as risks for non-relapse mortality.[7] Malnutrition is another critical factor, and tools like the Patient-Generated Subjective Global Assessment (PG-SGA) can identify patients in need of early nutritional intervention. We previously demonstrated that TGF-beta-enriched nutritional support improved outcomes, including reduced malnutrition, severe GI aGVHD, and infection, and improved survival.[8]
Furthermore, a slower pharmacokinetic profile of the agents used in the conditioning regimen in older patients may play a significant role. In particular, the pharmacokinetic variability of treosulfan has been documented in pediatric populations, where age-related differences in clearance are evident. However, data on adults and the elderly remain limited, and the influence of age on treosulfan pharmacokinetics in these groups is not yet fully understood.[9]
Microbiome integrity is another critical determinant of GI health. Conditioning, antibiotics, and dietary changes disrupt the microbiota, affecting outcomes.[10] Faraci et al. identified microbial signatures linked to aGVHD in pediatric patients. Changes included reduced Gammaproteobacteria and increased Alphaproteobacteria and Deltaproteobacteria.[11] Colonization by ESBL-producing bacteria is associated with dysbiosis, favoring genera like Bifidobacterium, Blautia, and Clostridium.[12] The fatal mycosis in our patient may also reflect interactions between occupational exposure, dysbiosis, and mucosal vulnerability.
Microbiome profiling offers a surrogate for intestinal health, informing personalized interventions to preserve or restore microbial balance, enhance immune recovery, and reduce GVHD and infection risk.
Conclusions
This case exemplifies the limitations of current pre-transplant assessments and highlights the need for a multidimensional evaluation of intestinal health. Future research should aim to identify subclinical gut impairment, refine conditioning regimens, and develop targeted interventions — including nutritional and microbiome-based strategies — to reduce GI complications and improve transplant outcomes.References
- Jansen SA, Nieuwenhuis EES, Hanash AM,
Lindemans CA. Challenges and opportunities targeting mechanisms of
epithelial injury and recovery in acute intestinal graft-versus-host
disease. Mucosal Immunol. 2022 Apr;15(4):605-619. https://doi.org/10.1038/s41385-022-00527-6 PMid:35654837 PMCid:PMC9259481
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier
BM, Maloney DG, Storer B. Hematopoietic cell transplantation
(HCT)-specific comorbidity index: a new tool for risk assessment before
allogeneic HCT. Blood. 2005 Oct 15;106(8):2912-9. https://doi.org/10.1182/blood-2005-05-2004 PMid:15994282 PMCid:PMC1895304
- Malagola M, Polverelli N, Martino M, et al.
Busulfan or Treosulfan Conditioning Platform for Allogeneic Stem Cell
Transplantation in Patients Aged >60 Y With Acute Myeloid
Leukemia/Myelodysplastic Syndrome: A Subanalysis of the GITMO AlloEld
Study. Transplant Direct. 2023 Feb 22;9(3):e1451. https://doi.org/10.1097/TXD.0000000000001451 PMid:36845852 PMCid:PMC9949804
- Wardill HR, Sonis ST, Blijlevens NMA, et al.
Prediction of mucositis risk secondary to cancer therapy: a systematic
review of current evidence and call to action. Support Care Cancer.
2020 Nov;28(11):5059-5073. https://doi.org/10.1007/s00520-020-05579-7 PMid:32592033
- Kuiken NSS, Rings EHHM, Blijlevens NMA, Tissing
WJE. Biomarkers and non-invasive tests for gastrointestinal mucositis.
Support Care Cancer. 2017 Sep;25(9):2933-2941. https://doi.org/10.1007/s00520-017-3752-2 PMid:28536886 PMCid:PMC5527064
- Pontoppidan PL, Jordan K, Carlsen AL, et al.
Associations between gastrointestinal toxicity, micro RNA and cytokine
production in patients undergoing myeloablative allogeneic stem cell
transplantation. Int Immunopharmacol. 2015 Mar;25(1):180-8. https://doi.org/10.1016/j.intimp.2014.12.038 PMid:25614225
- Gjærde LK, Ruutu T, Peczynski C, et al. The impact
of pre-transplantation diabetes and obesity on acute graft-versus-host
disease, relapse and death after allogeneic hematopoietic cell
transplantation: a study from the EBMT Transplant Complications Working
Party. Bone Marrow Transplant. 2024 Feb;59(2):255-263. https://doi.org/10.1038/s41409-023-02154-6 PMid:38062242 PMCid:PMC10849948
- Morello E, Brambilla G, Bernardi S, et al.
Nutritional intervention with TGF-beta enriched food for special
medical purposes (TGF-FSMP) is associated with a reduction of
malnutrition, acute GVHD, pneumonia and may improve overall survival in
patients undergoing allogeneic hematopoietic stem transplantation.
Transpl Immunol. 2023 Dec;81:101954. https://doi.org/10.1016/j.trim.2023.101954 PMid:37931667
- Romański M, Wachowiak J, Główka FK. Treosulfan
Pharmacokinetics and its Variability in Pediatric and Adult Patients
Undergoing Conditioning Prior to Hematopoietic Stem Cell
Transplantation: Current State of the Art, In-Depth Analysis, and
Perspectives. Clin Pharmacokinet. 2018 Oct;57(10):1255-1265. https://doi.org/10.1007/s40262-018-0647-4 PMid:29557088 PMCid:PMC6132445
- Malard F, Jenq RR. The Microbiome and Its Impact
on Allogeneic Hematopoietic Cell Transplantation. Cancer J. 2023
Mar-Apr;29(2):75-83. https://doi.org/10.1097/PPO.0000000000000645 PMid:36957977 PMCid:PMC10037670
- Faraci M, Bonaretti C, Dell'Orso G, et al.
Association between oral and fecal microbiome dysbiosis and treatment
complications in pediatric patients undergoing allogeneic hematopoietic
stem cell transplantation. Sci Rep. 2024 Mar 20;14(1):6708. https://doi.org/10.1038/s41598-024-55690-6 PMid:38509104 PMCid:PMC10954761
- Corcione S, Ferrocino I, Lupia T, et al. Influence
of ESBL colonization status on gut microbiota composition during
allogenic hematopoietic stem cell transplantation. Sci Rep. 2025 Jan
8;15(1):1275. https://doi.org/10.1038/s41598-025-85128-6 PMid:39779737 PMCid:PMC11711636