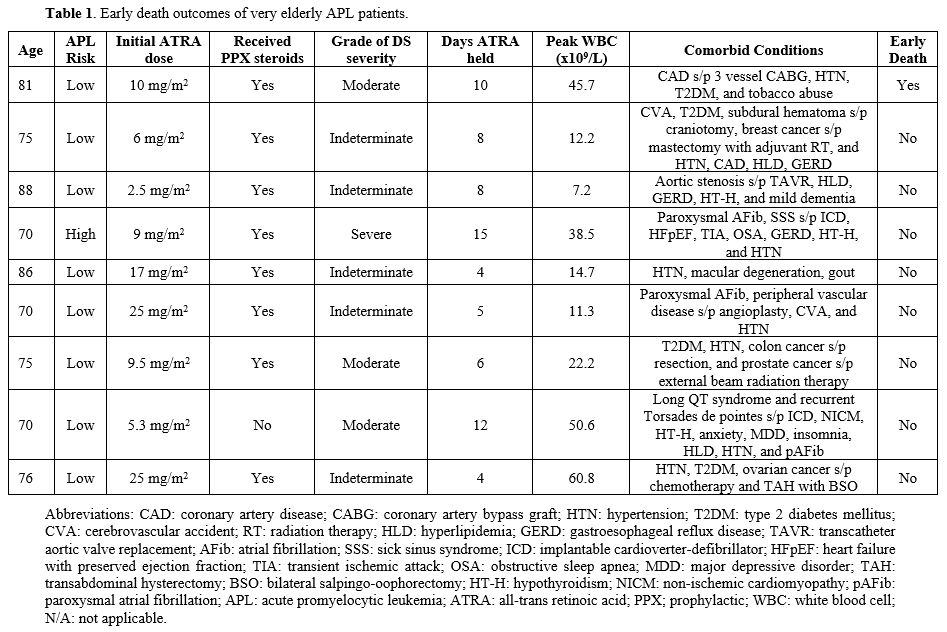
A retrospective analysis was conducted with institutional review board (IRB) approval of all elderly APL patients treated between 2017 and 2023 at Georgia Cancer Center in Augusta, GA. Screening was performed for all APL patients aged 70 years or older. Nine patients were identified and are summarized (Table 1). The patient population was between 70 and 88 years old, with a median age of 75 years. One female patient was classified as high risk and presented with white blood cell (WBC) of 31.7 x 109/L, while the rest were considered low risk with WBC <10 x 109/L. Due to age and significant comorbidities, all nine received dose-reduced ATRA (equal to or less than 25 mg/m2/day). ATRA was started as soon as APL was suspected, and patients were treated according to a simplified algorithm that emphasized the identification and prevention of disease and treatment of associated complications.[11,12]
Prednisone at 0.5 mg/kg was started at diagnosis in all low-risk patients except one, and dexamethasone 10 mg twice a day was started in the one high-risk patient per previously published protocols.[13] ATO was added after 10-14 days of therapy at a dose of 0.15 mg/kg daily or less if the patient was stable and deemed able to tolerate it. Cytoreductive agents, cytarabine and hydroxyurea, were used for hyperleukocytosis. Weights were obtained daily, and aggressive diuresis was performed to maintain patients at baseline weight. At the first sign of DS, ATRA was held, and the corticosteroid dose increased.
One early death was observed in the group. The patient was an 81-year-old Caucasian male with low-risk APL who elected to halt treatment and pursue hospice on day 17 of therapy and passed away on day 18. He was diagnosed with moderate DS on day 15 and reported having significant abdominal pain, dyspnea, and fever. He had several comorbidities, including coronary artery disease status-post 3-vessel coronary artery bypass graft, hypertension, and type 2 diabetes mellitus. Overall, the early death rate was noted to be 1/9 (11%) in this group, which is significantly lower than what has been reported in existing literature.[3,5] DS was noted to play a significant role in the care of all nine elderly APL patients. The severity of DS was graded according to the model proposed by the Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) group (Table 1).[14,15] Diagnosis of DS was made according to the presence of dyspnea, unexplained fever, weight gain greater than 5 kg, unexplained hypotension, acute renal failure, and a chest radiograph demonstrating pulmonary infiltrates or pleuropericardial effusion.
Patients with alternative explanations for the above signs or symptoms, such as septic shock, pneumonia, or heart failure, were not considered to have DS. If four or more of the above signs or symptoms were present, the patient was diagnosed as having severe DS. If two or three of the above signs or symptoms were present, the patient was classified as having moderate DS. If one of the above signs or symptoms were present with no other alternative explanation, the patient was classified as indeterminate DS. ATRA was held for different lengths of time throughout each patient’s hospitalization due to DS. This was seen despite the use of prophylactic steroids in eight out of the nine patients (Table 1). Several patients had their ATRA dose reduced further due to concern for recurrence of DS during induction therapy. Out of the nine patients treated, one was noted to have severe DS. This patient had an initial ATRA dose of 9 mg/m2/day and required multiple dose reductions. ATRA was held for a total of 15 days due to DS in this patient. Of note, the patient also required admission to the Intensive Care Unit, with a length of stay of 2 days.
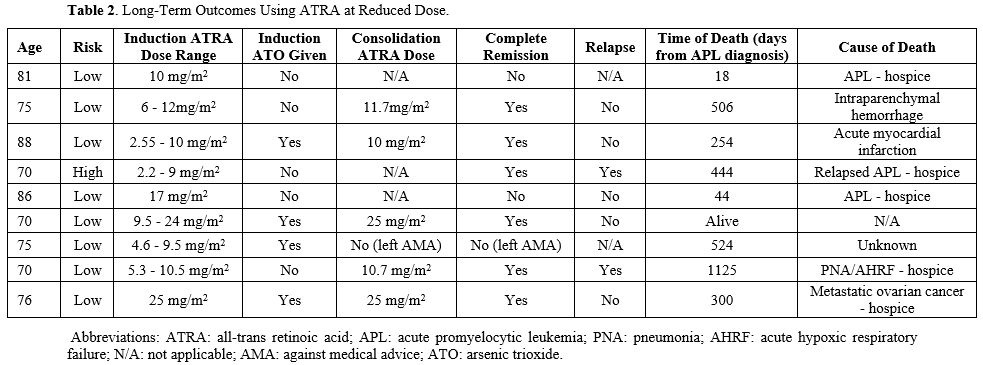
Maximum WBC count during induction therapy has been shown to correlate with both DS and early death, so this was also explored further.[14] The patients with moderate and severe DS had a WBC count that ranged between 22.2 and 50.6 x 109/L. It was also interesting that the one early death was noted in an 81-year-old patient with moderate DS with a maximum WBC count of 45.7 x 109/L. These findings support what previous studies have shown regarding the correlation between maximum WBC and DS as well as early death.[16] ATRA at a reduced dose was also given for consolidation (Table 2). Five out of the nine patients were able to receive further consolidation therapy, and all of them achieved complete hematologic and molecular remission. One patient was not a candidate for consolidation due to performance status and was later noted to have relapsed disease. Of the five patients who received induction and consolidation, one patient relapsed and continued therapy for several years.
Early death amongst APL patients, especially the elderly, remains a critical issue despite significant advances in therapy over the past 20 years. Clinical trials often exclude elderly patients due to age being a negative risk factor, so it is unclear what the most appropriate strategy should be regarding induction therapy. As patients age, the prevalence of other comorbidities increases, and that results in a greater chance of complications with induction therapy, suggesting that a more individualized approach may be necessary. From our earlier experiences, we learned that elderly patients tolerate standard doses of ATRA poorly, with increased deaths due to DS, and so subsequently decreased the doses, which showed improved outcomes.[11,12] One of the areas of focus we looked at was how elderly APL patients responded to lower doses of ATRA compared to the standard regimen of 45 mg/m2. Interestingly, only one early death was noted, and this patient decided to pursue hospice due to negative effects from DS. It is also important to note that all the patients were affected by DS, despite receiving lower doses of ATRA as well as prophylactic steroids. These findings suggest that DS may be playing a greater role in influencing early death rates amongst elderly patients than previously believed. One of the ways that the severity of DS can be targeted and early death rates decreased is by administering lower doses of ATRA during induction therapy and withholding the drug at the first sign of DS. We suggest 25 mg/m2 of ATRA in patients above 70 years of age, and a further decrease to 10 mg/m2 in the very elderly with significant comorbidities. Single agent ATRA during induction and adding ATO later in induction, if there is no evidence of DS or leukocytosis, or only during consolidation, is an approach we used. We believe that this area needs to be explored further and could be crucial to improving survival amongst elderly APL patients.
References
- Lo-Coco F, Avvisati G, Vignetti M, Thiede C,
Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E,
Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M,
Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH,
Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lübbert M,
Hänel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M,
Ciceri F, Finizio O, Venditti A, Fabbiano F, Döhner K, Sauer M, Ganser
A, Amadori S, Mandelli F, Döhner H, Ehninger G, Schlenk RF, Platzbecker
U; Gruppo Italiano Malattie Ematologiche dell'Adulto; German-Austrian
Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic
acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J
Med. 2013 Jul 11;369(2):111-21. https://doi.org/10.1056/NEJMoa1300874 PMid:23841729
- Jácomo
RH, Melo RA, Souto FR, de Mattos ER, de Oliveira CT, Fagundes EM,
Bittencourt HN, Bittencourt RI, Bortolheiro TC, Paton EJ, Bendlin R,
Ismael S, Chauffaille Mde L, Silva D, Pagnano KB, Ribeiro R, Rego EM.
Clinical features and outcomes of 134 Brazilians with acute
promyelocytic leukemia who received ATRA and anthracyclines.
Haematologica. 2007 Oct;92(10):1431-2. https://doi.org/10.3324/haematol.10874 PMid:18024380
- Lehmann
S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Möllgård L, Derolf AR,
Stockelberg D, Tidefelt U, Wahlin A, Wennström L, Höglund M, Juliusson
G. Continuing high early death rate in acute promyelocytic leukemia: a
population-based report from the Swedish Adult Acute Leukemia Registry.
Leukemia. 2011 Jul;25(7):1128-34. doi: 10.1038/leu.2011.78. Epub 2011
Apr 19. https://doi.org/10.1038/leu.2011.78 PMid:21502956
- Park
JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, Altman
JK, Douer D, Rowe JM, Tallman MS. Early death rate in acute
promyelocytic leukemia remains high despite all-trans retinoic acid.
Blood. 2011 Aug 4;118(5):1248-54. https://doi.org/10.1182/blood-2011-04-346437 PMid:21653939 PMCid:PMC3790946
- Lengfelder
E, Hanfstein B, Haferlach C, Braess J, Krug U, Spiekermann K, Haferlach
T, Kreuzer KA, Serve H, Horst HA, Schnittger S, Aul C, Schultheis B,
Erben P, Schneider S, Müller-Tidow C, Wörmann B, Berdel WE, Sauerland
C, Heinecke A, Hehlmann R, Hofmann WK, Hiddemann W, Büchner T; German
Acute Myeloid Leukemia Cooperative Group (AMLCG). Outcome of elderly
patients with acute promyelocytic leukemia: results of the German Acute
Myeloid Leukemia Cooperative Group. Ann Hematol. 2013 Jan;92(1):41-52.
Epub 2012 Oct 23. https://doi.org/10.1007/s00277-012-1597-9 PMid:23090499 PMCid:PMC3536950
- Jillella
AP, Arellano ML, Gaddh M, Langston AA, Heffner LT, Winton EF, McLemore
ML, Zhang C, Caprara CR, Simon KS, Bolds SL, DeBragga S, Karkhanis P,
Krishnamurthy SH, Tongol J, El Geneidy MM, Pati A, Gerber JM, Grunwald
MR, Cortes J, Bashey A, Stuart RK, Kota VK. Comanagement Strategy
Between Academic Institutions and Community Practices to Reduce
Induction Mortality in Acute Promyelocytic Leukemia. JCO Oncol Pract.
2021 Apr;17(4):e497-e505. Epub 2020 Oct 30. https://doi.org/10.1200/OP.20.00395 PMCid:PMC8202058
- Jin
B, Zhang Y, Hou W, Cao F, Lu M, Yang H, Tian X, Wang Y, Hou J, Fu J, Li
H, Zhou J. Comparative analysis of causes and predictors of early death
in elderly and young patients with acute promyelocytic leukemia treated
with arsenic trioxide. J Cancer Res Clin Oncol. 2020
Feb;146(2):485-492. Epub 2019 Nov 4. https://doi.org/10.1007/s00432-019-03076-x PMid:31686248 PMCid:PMC11804490
- Testi
AM, D'Angiò M, Locatelli F, Pession A, Lo Coco F. Acute Promyelocytic
Leukemia (APL): Comparison Between Children and Adults. Mediterr J
Hematol Infect Dis. 2014 Apr 15;6(1):e2014032. https://doi.org/10.4084/mjhid.2014.032 PMid:24804005 PMCid:PMC4010611
- Ortega
JJ, Madero L, Martín G, Verdeguer A, García P, Parody R, Fuster J,
Molines A, Novo A, Debén G, Rodríguez A, Conde E, de la Serna J,
Allegue MJ, Capote FJ, González JD, Bolufer P, González M, Sanz MA;
PETHEMA Group. Treatment with all-trans retinoic acid and anthracycline
monochemotherapy for children with acute promyelocytic leukemia: a
multicenter study by the PETHEMA Group. J Clin Oncol. 2005 Oct
20;23(30):7632-40. https://doi.org/10.1200/JCO.2005.01.3359 PMid:16234524
- Castaigne
S, Lefebvre P, Chomienne C, Suc E, Rigal-Huguet F, Gardin C, Delmer A,
Archimbaud E, Tilly H, Janvier M, et al. Effectiveness and
pharmacokinetics of low-dose all-trans retinoic acid (25 mg/m2) in
acute promyelocytic leukemia. Blood. 1993 Dec 15;82(12):3560-3. https://doi.org/10.1182/blood.V82.12.3560.3560 PMid:8260694
- Jillella
AP, Lee SJ, Altman JK, Luger SM, Tallman MS, Foran JM, Bradshaw D, Law
LY, Bryan LJ, Abou Zahr A, Begna KH, Perl AE, Vadakara JJL, Qamar R,
Bergan RC, Fisch MJ, Carlos RC, Wagner LI, Kota VK, Litzow MR. Academic
Community Partnership in Acute Promyelocytic Leukemia and Early
Mortality: The ECOG-ACRIN EA9131 Trial. JAMA Oncol. 2025 Feb
27:e247033. https://doi.org/10.1001/jamaoncol.2024.7033 PMid:40014329 PMCid:PMC11869096
- Jillella
AP, Karkhanis P, Sharma R, Bolds S, Shrestha A, Sitchenko K,
Jonnalagadda V, Gaddh M, Bernal-Mizrachi L, Heffner LT, Winton EF,
McLemore ML, Langston A, Galipeau J, Bodó I, Al-Kadhimi Z, Bashey A,
Stuart RK, Vidito S, Pati A, Gerber JM, Grunwald MR, Bradley KT, Tongol
J, El-Geneidy M, Khoury HJ, Arellano M, Kota V. A Multicenter
Prospective Study Utilizing a Simplified Treatment Algorithm
Complemented By Expert Support Decreases Induction Mortality and
Improves Survival in Acute Promyelocytic Leukemia (APL). Results of the
APL Trial in Georgia, South Carolina and Neighboring States. Blood
2016; 128 (22): 2793. https://doi.org/10.1182/blood.V128.22.2793.2793
- Lo-Coco
F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, Paoloni F,
Fioritoni G, Ferrara F, Specchia G, Cimino G, Diverio D, Borlenghi E,
Martinelli G, Di Raimondo F, Di Bona E, Fazi P, Peta A, Bosi A, Carella
AM, Fabbiano F, Pogliani EM, Petti MC, Amadori S, Mandelli F; Italian
GIMEMA Cooperative Group. Front-line treatment of acute promyelocytic
leukemia with AIDA induction followed by risk-adapted consolidation for
adults younger than 61 years: results of the AIDA-2000 trial of the
GIMEMA Group. Blood. 2010 Oct 28;116(17):3171-9. https://doi.org/10.1182/blood-2010-03-276196 PMid:20644121
- Montesinos
P, Sanz MA. The differentiation syndrome in patients with acute
promyelocytic leukemia: experience of the pethema group and review of
the literature. Mediterr J Hematol Infect Dis. 2011;3(1):e2011059. Epub
2011 Dec 4. https://doi.org/10.4084/mjhid.2011.059 PMid:22220256 PMCid:PMC3248336
- Stahl M, Tallman MS. Differentiation syndrome in acute promyelocytic leukaemia. Br J Haematol. 2019 Oct;187(2):157-162. https://doi.org/10.1111/bjh.16151 PMid:31410848
- Wen J, Xu F, Zhou Q, Shi L, Liu Y, Yue J, Zhang Y, Liang X. Effects of peripheral blood leukocyte count and tumor necrosis factor-alpha on early death in acute promyelocytic leukemia. BMC Cancer. 2023 Jan 7;23(1): https://doi.org/10.1186/s12885-022-10499-2 PMid:36611025 PMCid:PMC9824944