Additionally, venetoclax combined with low-dose cytarabine (LDAC) has shown superior efficacy over traditional regimens, with significant improvements in overall survival and complete remission rates.[7] Despite these advancements, challenges remain, particularly in patients with adverse genetic profiles such as TP53 mutations.[8,9] Overall, while venetoclax and azacitidine represent a significant advancement in the treatment landscape for AML, particularly for elderly and unfit patients,[10] further research is needed to optimize these regimens and address the high relapse rates associated with this disease.[4-6]
The primary objective of this study was to evaluate the overall response rate (ORR) of venetoclax plus azacitidine in elderly patients with relapsed AML. This population currently faces poor outcomes and limited therapy options. In addition, we also aimed to determine overall survival (OS), event-free survival (EFS), and the safety profile of this combination therapy as secondary objectives. Furthermore, we explored the prognostic impact of baseline blood-borne factors - such as lactate dehydrogenase (LDH), C-reactive protein (CRP), and β2-microglobulin - to gain deeper insight into potential biomarkers that may guide risk stratification and patient selection.
Methods
Study Design and Setting. This single-center, retrospective study was conducted at the Quzhou Affiliated Hospital of Wenzhou Medical University. We reviewed medical records of consecutive patients with relapsed AML who were treated with a combination of venetoclax and azacitidine between January 2018 and December 2022. The study was authorized by the Medical Ethics Committee of the Quzhou Affiliated Hospital of Wenzhou Medical University, and informed consent was obtained from all participants.Inclusion Criteria: 1), age≥65 years at the time of relapse diagnosis; 2), Histologically confirmed AML according to World Health Organization (WHO) criteria; 3), Relapsed disease after at least one prior line of therapy, as documented by bone marrow (BM) biopsy; 4), treatment with venetoclax plus azacitidine for at least one cycle; 5), Adequate medical records allowing assessment of baseline characteristics, treatments, and outcomes.
Exclusion Criteria: 1), Concurrent participation in another investigational trial that included any other experimental agent; 2), Insufficient data on treatment response or survival outcomes due to incomplete medical records; 3), Known active central nervous system (CNS) involvement of leukemia; 4), Significant liver or renal dysfunction unrelated to AML, which precluded the administration of venetoclax or azacitidine, as per treating physician judgment.
Patient selection & fitness assessment. In routine practice, azacitidine plus venetoclax was selected for patients considered unsuitable for intensive salvage based on one or more of the following: performance status, comorbidity burden and organ function, antecedent therapy intensity/exposure, and patient preference after multidisciplinary discussion. We abstracted comorbidities data (hypertension, diabetes, coronary/cerebrovascular disease, chronic kidney or lung disease, hepatic disease, heart failure, prior malignancy) from the electronic record. We summarized comorbidity burden using the HCT‑CI where derivable.
Treatment Protocol. Venetoclax: To mitigate tumor lysis syndrome (TLS), venetoclax was ramped over 3 days when combined with azacitidine: 100 mg on Day 1, 200 mg on Day 2, and 400 mg on Day 3, followed by 400 mg once daily thereafter in each 28‑day cycle. Dose modifications followed label and guideline recommendations for drug–drug interactions: with posaconazole (strong CYP3A inhibitor), venetoclax was reduced to 10, 20, 50, and 70 mg on Days 1–4 during initiation, then 70 mg once daily while co‑administered; with other strong CYP3A inhibitors, 10, 20, 50, and 100 mg on Days 1–4 during initiation, then 100 mg once daily; with moderate CYP3A inhibitors, the venetoclax dose was reduced by ≥50%. TLS prophylaxis (hydration/urate‑lowering) and laboratory monitoring were provided per institutional practice. Azacitidine: Azacitidine was administered at 75 mg/m2 per day subcutaneously or intravenously for seven consecutive days per 28-day cycle, following the standard institutional protocol for hypomethylating agents. Dose modifications were considered according to hematologic recovery and toxicity profiles.
Supportive Care: All patients received supportive care measures per institutional standards, including prophylactic antimicrobial agents (antibacterial, antifungal) when indicated, transfusions for symptomatic cytopenias, and growth factor support (e.g., granulocyte colony-stimulating factor) based on physician discretion.
Data Collection. Baseline Variables: Clinical and laboratory data were extracted from electronic medical records. Baseline evaluations included demographics (age, sex), comorbidities, and Eastern Cooperative Oncology Group (ECOG) performance status, as well as a detailed prior treatment history (number and type of previous regimens, remission durations). Key laboratory values such as complete blood count (CBC), lactate dehydrogenase (LDH), C-reactive protein (CRP), β2-microglobulin, and ferritin. FLT3‑ITD status was captured as present/absent. Allelic ratio was not uniformly available, and risk terminology follows ELN‑2017 in our dataset.
Patients were typically evaluated for response after each cycle using bone marrow aspirate/biopsy and peripheral blood counts.
Allogeneic hematopoietic stem‑cell Transplantation: History of allogeneic hematopoietic stem‑cell transplantation (allo‑HSCT) prior to the index relapse was abstracted from the medical record and analyzed as a baseline variable; we compared ORR (CR+CRi) by prior allo‑HSCT status (yes vs no) as an exploratory subgroup. We recorded post-response subsequent therapies, including allogeneic hematopoietic stem-cell transplantation (allo-SCT) performed after HMA+venetoclax. For patients proceeding to allo‑SCT, we captured response status at transplant (CR vs CRi) and the number of cycles administered before transplant.
Outcome Measures. Primary Endpoint: ORR was defined as the proportion of patients achieving either CR or CRi, based on the 2017 European LeukemiaNet (ELN) criteria or institutional criteria.
Secondary Endpoints: OS was defined as the survival time from the start of venetoclax-azacitidine to death from any cause or last follow-up. EFS was defined as the survival time from treatment initiation to disease progression, relapse, or death.
Safety and Tolerability: Incidence and severity of adverse events (AEs), graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE).
Prognostic Factors: Evaluation of baseline variables, including blood-borne biomarkers, molecular abnormalities, and performance status, to identify independent predictors of survival.
Statistical Analysis. All statistical analyses were conducted using R (version 4.4). Descriptive statistics were presented as medians (interquartile ranges) or means (standard deviations) for continuous variables, and as frequencies (percentages) for categorical variables. Group comparisons were performed using Fisher’s exact test or chi-square test for categorical variables and the Mann-Whitney U test or Student’s t-test for continuous variables, as appropriate. OS and EFS were estimated using the Kaplan-Meier method and compared by the log-rank test. A Cox proportional hazards model was used to identify independent predictors of survival. Variables that yielded a p-value <0.1 in univariate analysis or were deemed clinically relevant were included in the multivariate model. Overall survival (OS) and event‑free survival (EFS) were estimated by the Kaplan–Meier method with 95% CIs and compared using the log‑rank test. To visualize the association between response and outcomes while reducing immortal‑time bias, we performed a 60‑day landmark analysis (end of Cycle 2): patients alive at Day 60 were grouped by achievement of CR/CRi by Day 60 versus no CR/CRi by Day 60, and OS/EFS were plotted from the landmark. Censoring followed standard conventions; number‑at‑risk tables are shown beneath each plot. Cox models are reported separately. p <0.05 was considered statistically significant.
Results
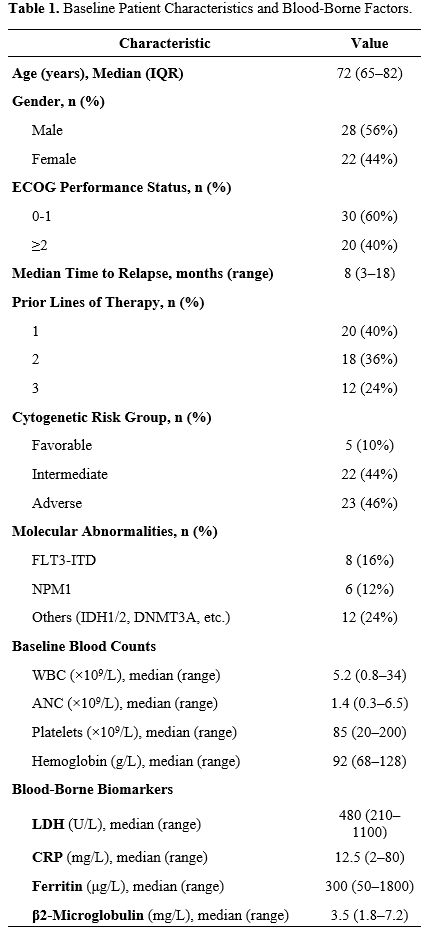
A total of 50 patients with relapsed AML were included in this analysis. The median age was 72 years (range, 65–82), and 56% (n=28) were male (Table 1). Among these patients, 40% (n=20) had an Eastern Cooperative Oncology Group (ECOG) performance status of ≥2 (Table 1). The majority presented with intermediate (44%) or adverse (46%) cytogenetic features, and 16% (n=8) were FLT3-ITD-positive (Table 1). Baseline laboratory values revealed elevated LDH in 40% of the cohort and high CRP levels in 28% (Table 1). Additional blood-borne factors - such as ferritin and β2-microglobulin - were available in most patients and are detailed in Table 1.Comorbidity burden was substantial in this ≥65‑year cohort. 44 out of 50 participants (88.0%) had at least one chronic condition, and 29/50 (58.0%) had two or more. Based on the HCT‑CI, 22/50 (44.0%) were in the 0–2 category, 18/50 (36.0%) in 3-4, and 10/50 (20.0%) in ≥5. The most common comorbidities were hypertension (31/50, 62.0%), diabetes (15/50, 30.0%), chronic kidney disease (12/50, 24.0%), coronary artery disease (11/50, 22.0%), and chronic lung disease (9/50, 18.0%); 7/50 (14.0%) had a prior malignancy. Consistent with selection for less‑intensive salvage, 20/50 (40.0%) had ECOG ≥2 at baseline (Supplementary Table S1).
A total of 20 participants (40.0%) had one prior line, 18/50 (36.0%) had two, and 12/50 (24.0%) had three. Prior exposures (non‑mutually exclusive) included intensive chemotherapy (30/50, 60.0%), hypomethylating agents (18/50, 36.0%), venetoclax (5/50, 10.0%), FLT3 inhibitors (6/50, 12.0%), and IDH1/2 inhibitors (3/50, 6.0%); 7/50 (14.0%) had undergone allo‑HSCT. At the index relapse, 30/50 (60.0%) were at first relapse, 14/50 (28.0%) at second, and 6/50 (12.0%) at ≥third or subsequent relapse. The duration of the most recent complete Remission before relapse was <6 months in 24/50 (48.0%), 6–12 months in 16/50 (32.0%), and >12 months in 10/50 (20.0%). These features contextualize the clinical rationale for a less‑intensive HMA+VEN approach (Supplementary Table S2).
Seven out of fifty (14.0%) patients had undergone allo‑HSCT before the index relapse. ORR was 4/7 (57.1%) in previously transplanted patients and 26/43 (60.5%) in those without prior transplant (Fisher’s exact p=1.00). Within the allo‑HSCT subgroup, CR and CRi occurred in 2/7 (28.6%) and 2/7 (28.6%), respectively; among patients without prior allo‑HSCT, CR was 18/43 (41.9%) and CRi was 8/43 (18.6%). Rates of PR, SD, and PD were 0/7 (0.0%), 1/7 (14.3%), and 2/7 (28.6%) in previously transplanted patients vs 5/43 (11.6%), 7/43 (16.3%), and 5/43 (11.6%) without prior transplant. While sample size limits power, these findings do not indicate a difference in response by prior allo‑HSCT status (Supplementary Table S3).
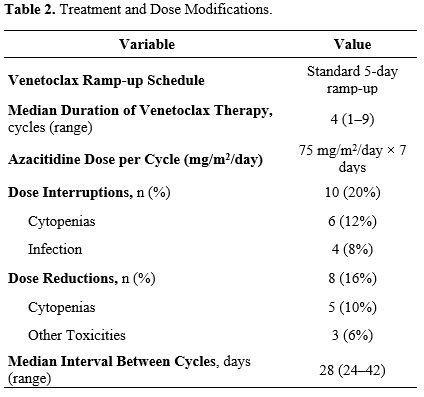
All participants received a ramp-up schedule of venetoclax over three days (100–400 mg daily) alongside azacitidine administered at 75 mg/m2 per day for seven days in each 28-day cycle (Table 2). The median number of treatment cycles was four (range, 1–9). Ten patients (20%) required at least one dose interruption due to cytopenias or infection, while 8 (16%) underwent dose reductions (Table 2). Despite these modifications, the majority (80%) were able to continue therapy without long-term interruptions (Table 2).
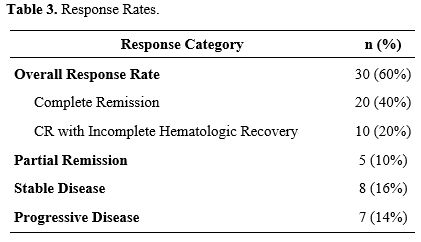
ORR was 60% (n=30), comprising 40% (n=20) CR and 20% (n=10) complete remissions with incomplete hematologic recovery (CRi) (Table 3). Partial Remission was documented in 5 patients (10%), while 8 (16%) had stable disease and 7 (14%) progressed during treatment (Table 3). Response rates appeared comparable across intermediate- and adverse-risk cytogenetic categories, although the sample size precluded definitive subgroup analysis (Table 3).
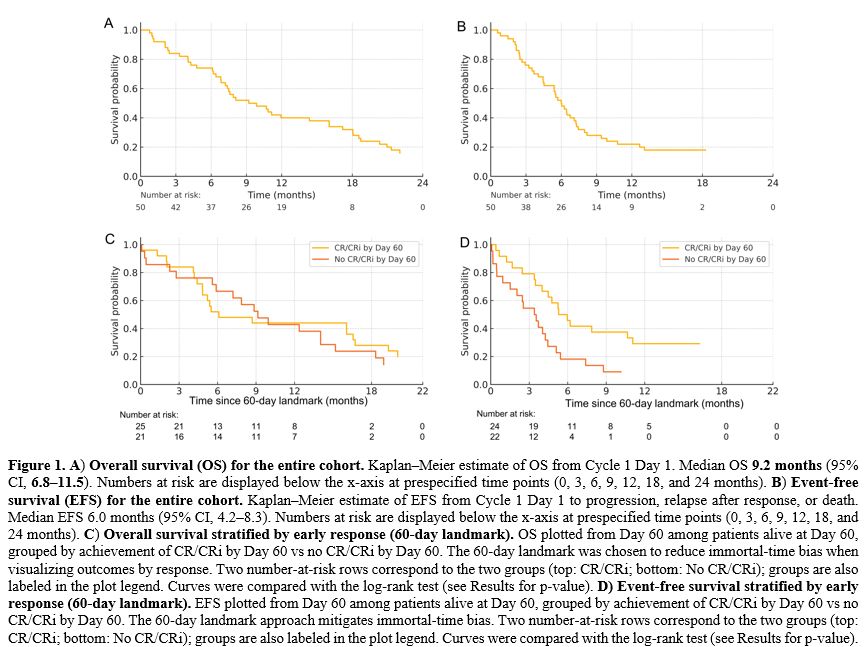
Median OS for the entire cohort was 9.2 months (95% CI, 6.8–11.5), and the median EFS was 6.0 months (95% CI, 4.2–8.3) (Table 4 and Figure 1). In a 60-day landmark analysis, patients who had achieved CR/CRi by Day 60 experienced longer OS compared with those without CR/CRi by Day 60 (Figure 1; log-rank p<0.05), consistent with the response associations described in the multivariable model. EFS curves showed a concordant separation favoring early responders (Figure 1). Together, these figures illustrate that achieving CR/CRi early during venetoclax + azacitidine is associated with more favorable time‑to‑event outcomes in this ≥65‑year relapsed AML cohort. Baseline coagulation data were available in 42/50 (84.0%) and supported ISTH DIC scoring in 40/50 (80.0%). Median (range) values were PT/INR 1.13 (0.97–1.47), aPTT 31.0 s (25–46), fibrinogen 2.3 g/L (1.0–5.2), and D‑dimer 1.6 mg/L FEU (0.2–6.4); 12/42 (28.6%) had D‑dimer >2.0 mg/L FEU. Overt DIC at treatment start was uncommon (5/40, 12.5%). Thirty‑day mortality was 2/50 (4.0%) overall and 1/5 (20.0%) among those with overt DIC; no statistically significant associations between DIC parameters and OS/EFS were detected in this small, retrospectively captured dataset (Supplementary Table S4).
 |
Table 4. Survival Outcomes. |
Univariate Cox analysis identified ECOG performance status ≥2, adverse cytogenetics, and elevated LDH (≥ upper limit of normal) as significant risk factors for poorer OS (p<0.05) (Table 5). In multivariate analysis, ECOG performance status (HR: 2.1; 95% CI, 1.3–3.4; p=0.002), adverse cytogenetics (HR: 1.8; 95% CI, 1.1–3.0; p=0.015), and high LDH (HR: 1.6; 95% CI, 1.0–2.6; p=0.048) remained independent predictors of survival (Table 6). Elevated β2-microglobulin demonstrated a trend toward worse outcomes (p=0.066), but did not reach statistical significance in the multivariate model (Table 6).
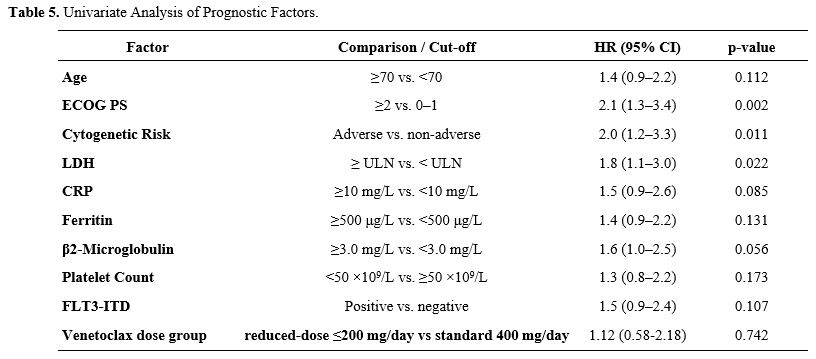 |
Table 5. Univariate Analysis of Prognostic Factors. |
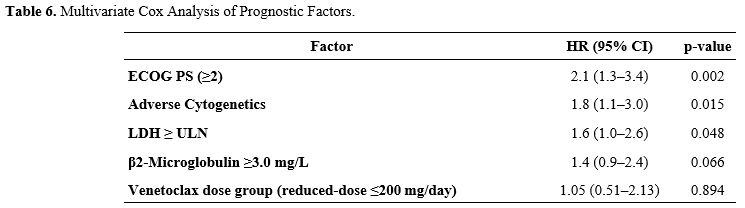 |
Table 6. Multivariate Cox Analysis of Prognostic Factors. |
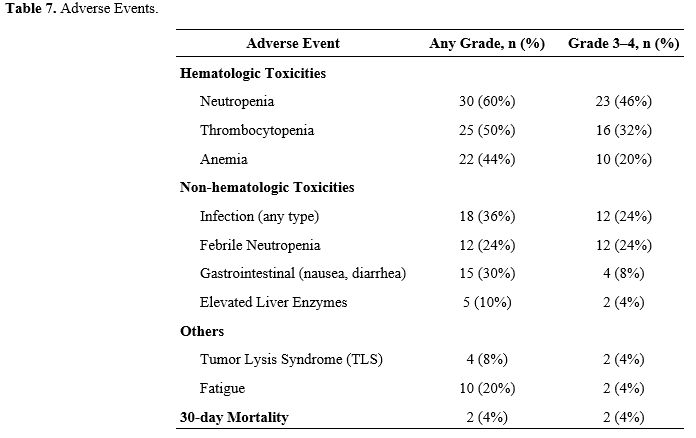
Treatment-related toxicities were common but largely manageable. As shown in Table 7, hematologic events were the most frequently reported, with grade 3–4 neutropenia in 46% (n=23) and thrombocytopenia in 32% (n=16). Non-hematologic adverse events included infections (36%), febrile neutropenia (24%), and gastrointestinal symptoms (30%). The 30-day treatment-related mortality rate was 4% (n=2). Supportive measures such as prophylactic antimicrobials and growth factor administration were employed according to institutional guidelines.
Discussion
Our study demonstrated that elderly patients with relapsed AML treated with venetoclax plus azacitidine achieved notable response rates, with the majority reaching either complete Remission or complete Remission with incomplete hematologic recovery. Additionally, both overall survival and event-free survival were clinically meaningful, suggesting the potential efficacy of this regimen in a population traditionally characterized by limited therapeutic options and poor prognoses. Several baseline blood-borne factors, including LDH, CRP, and β2-microglobulin, emerged as relevant prognostic markers, providing insight into how patient-specific biology may influence treatment outcomes. Taken together, these findings indicate that venetoclax plus azacitidine not only offers a promising treatment avenue for this vulnerable population but also underscores the importance of prognostic stratification in optimizing clinical decision-making.In our single-center cohort of elderly patients with relapsed AML, venetoclax plus azacitidine yielded encouraging response rates and survival outcomes that align closely with previous clinical trials and retrospective analyses.[11-13] Our ORR was similar to the high remission rates observed in a phase II trial[10] and in other studies focusing on older or unfit patients.[14] Although direct head-to-head comparisons are limited by differing definitions of relapse and Remission, our findings echo the promising results reported for both newly diagnosed and relapsed/refractory AML.[15,16] Notably, our patients also exhibited manageable toxicity, which is consistent with emerging real-world data.[13,17] These similarities affirm that the combination of venetoclax and hypomethylating agents can offer significant clinical benefits in elderly, high-risk settings.[18]
In our analysis, baseline blood‑borne biomarkers - including LDH, CRP, and β2‑microglobulin - emerged as clinically relevant indicators of outcome. LDH ≥ the institutional ULN independently predicted shorter overall survival, and elevated CRP and β2‑microglobulin showed adverse trends. These routinely available measures may complement ECOG performance status and cytogenetic risk to refine selection, counseling, and monitoring for older adults treated with venetoclax plus azacitidine. Prospective studies with standardized genomic panels (and contemporary ELN risk frameworks) will be useful to validate integrated clinical‑biomarker models in the relapsed setting.
Allogeneic hematopoietic stem cell transplantation (alloSCT) remains the only potentially curative therapy for most patients with relapsed AML, owing to a graft versus leukemia effect that chemotherapy alone cannot reproduce.[19] In our ≥ 65-year relapsed AML cohort treated with venetoclax plus azacitidine, we observed an ORR of 60% (CR 40%, CRi 20%) with median OS 9.2 months and median EFS 6.0 months, and patients achieving CR/CRi experienced longer OS - reinforcing the clinical importance of attaining Remission before consolidation. These remissions can serve as an effective bridge to transplant for selected older adults, consistent with multicenter cohorts in relapsed AML showing that venetoclax based salvage enables a meaningful proportion of responders to proceed to allo SCT with acceptable peri transplant safety,[20] while reduced intensity or non myeloablative conditioning improves feasibility by lowering treatment related mortality in older patients.[21] Because post-transplant outcomes are strongly influenced by pre-HCT measurable residual disease status, integrating measurable residual disease assessment into venetoclax-based salvage pathways may help optimize relapse risk and survival after allo-SCT in relapsed AML.[22,23]
Our real-world, single-center experience provides practical insights for a vulnerable, often underrepresented elderly AML population, and our biomarker analysis adds novelty to the existing literature. However, the retrospective design, relatively small sample size, and single-center scope may limit the generalizability of our findings, and incomplete biomarker data in some cases, along with the absence of a randomized control arm, constrain the strength of our conclusions. Future work should involve larger, prospective, or multicenter studies to validate these results, explore novel therapeutic combinations and more robust biomarker profiling, and ultimately advance personalized medicine strategies to optimize treatment outcomes.
Conclusions
Our findings suggest that venetoclax plus azacitidine represents a promising therapeutic option for elderly, relapsed AML patients who typically have few viable treatment alternatives. The inclusion of comprehensive biomarker analyses, such as LDH, CRP, and β2-microglobulin, provides valuable prognostic insights that may help tailor therapy to individual patient risk profiles.Ethical Approval
The study was authorized by the Medical Ethics Committee of the Quzhou Affiliated Hospital of Wenzhou Medical University, and informed consent was obtained from all participants.Data availability statement
Data sets generated during the current study are available from the corresponding author on reasonable request.References
- Gopishetty,
S., Jaiyesimi, I., & Ezekwudo, D. (2024). Outcomes of Acute Myeloid
Leukemia Patients Aged 60 Years and Above: An Analysis of SEER Data.
Blood, 144(Supplement 1), 7924. https://doi.org/10.1182/blood-2024-212095
- Shimanovskaya,
L. T., Misyurina, E. N., Baryakh, E. A., Zhelnova, E. I., Yatskov, K.
V., Chudnova, T. S., Tolstykh, T. N., & Gagloeva, D. E. (2024).
Treatment of patients with acute myeloid leukemia in the older age
group: Experience of City Clinical Hospital No. 52. Oncohematology,
19(4), 14-22. https://doi.org/10.17650/1818-8346-2024-19-4-14-22
- Meng,
S. H. (2024). Acute Myeloid Leukemia Overview With Focus On Small
Molecules Treatment. Theoretical and Natural Science, 73(1), 38- 45. https://doi.org/10.54254/2753-8818/2024.18871
- Gilbert,
J. S., Connor, M. K., Bosma, G., McMahon, C. M., Amaya, M. L., Gutman,
J. A., Schwartz, M., Kent, A., Abbott, D., & Pollyea, D. A. (2024).
Efficacy and Molecular Predictors of Response and Survival for
Venetoclax/Azacitidine Therapy in Relapsed or Refractory Acute Myeloid
Leukemia. Blood, 144(Supplement 1), 4266. https://doi.org/10.1182/blood-2024-199862
- Li,
X., Li, M., Cui, W., Chen, X., Yu, Y., Chen, J., Zhang, X., Cui, Q.,
Wu, D., & Tang, X. (2024). How to Improve the Efficacy of
Venetoclax and Azacitidine(VA)for Newly Diagnosed Acute Myeloid
Leukemia? a Prospective, Single-Center, Single-Arm, Phase 2 Trial for
New Diagnosed AML. Blood, 144(Supplement 1), 4284. https://doi.org/10.1182/blood-2024-208981
- Roboz,
G. J., Aribi, A., Ochsenbein, A. F., Riether, C., Stuart, M. J.,
Mitchell, E., Changela, K., Adam, G. K., Boyiadzis, M., Smith, C.,
& Pabst, T. (2024). Trial in Progress: A Multicenter, Open Label,
Randomized, Phase 2 Study of Venetoclax and Azacitidine Plus
Cusatuzumab Versus Venetoclax and Azacitidine Alone in Newly Diagnosed
AML Patients Who Are Not Candidates for Intensive Therapy. Blood,
144(Supplement 1), 1504.2. https://doi.org/10.1182/blood-2024-202648
- Wang,
K., Pokima, N., Keesari, P. R., El-gharib, K., Widjaja, M., &
El‐Sayegh, S. (2024). The Efficacy of Venetoclax + LDAC and CPX-351
over Traditional Regimen in Older Patients with AML Not Eligible for
Intensive Therapy: A Meta-Analysis and Systematic Review. Blood,
144(Supplement 1), 6073. https://doi.org/10.1182/blood-2024-208801
- Elbeih,
A., Ghosoun, N., McCullough, K. B., Johnson, I., Abdelmagid, M.,
Al-Kali, A., Alkhateeb, H. B., Begna, K., Elliott, M. A., Mangaonkar,
A., Matin, A., Saliba, A. N., Hefazi, M., Litzow, M. R., Hogan, W. J.,
Shah, M. V., Patnaik, M. M., Pardanani, A. D., Badar, T., …
Gangat, N. (2024). Predictors of relapse and post-relapse outcomes in
patients with newly-diagnosed acute myeloid leukemia treated with
venetoclax + hypomethylating agent. Blood, 144(Suppl. 1), 2852. https://doi.org/10.1182/blood-2024-209015
- Bołkuń,
Ł., Strzała, J., Czemerska, M., Budziszewska, B. K., Gołoś, A.,
Hołownia, A., Knopińska‐Posłuszny, W., Lech‐Marańda, E., Piszcz, J.,
Hałka, J., & Wierzbowska, A. (2024). Standard Non-Intensive
Chemotherapy Vs Venetoclax-Azacytidine (Ven-AZA) for Unfit AML Patients
with Therapy-Related Acute Myeloid Leukaemia (t-AML) - a Retrospective
Study of Polish Adult Leukemia Group (PALG). Blood, 144(Supplement 1),
6056. https://doi.org/10.1182/blood-2024-207883
- Cristiano
A., Palmieri R., Fabiani E., Ottone T., Divona M., Savi A., Buccisano
F., Maurillo L., Tarella C., Arcese W., Voso M.T.The
venetoclax/azacitidine combination targets the disease clone in Acute
Myeloid Leukemia, being effective and safe in a patient with COVID.
Mediterr J Hematol Infect Dis 2022, 14(1): e2022041 https://doi.org/10.4084/MJHID.2022.041 PMid:35615323 PMCid:PMC9083951
- Medawar,
G., Tharakan, S., Sastow, D., Lok, V., Hahn, S. B., Perez, P., Demers,
A., Swoboda, D. M., Tremblay, D., & Coltoff, A. (2024). Extended Vs
Standard Hypomethylating Agent Dosing in Combination with Venetoclax in
Newly Diagnosed Acute Myeloid Leukemia. Blood, 144(Supplement 1), 2904.
https://doi.org/10.1182/blood-2024-209414
- Manda,
S., Anz, B., Benton, C., Broun, E. R., Yimer, H. A., Renshaw, J. S.,
Geils, G., Berdeja, J. G., Cruz, J., Melear, J. M., Fanning, S. R.,
Fletcher, L., Li, Y., Duan, Y., Werner, M., Potluri, J., Pai, M. V.,
& Donnellan, W. B. (2024). A phase 3b study of venetoclax and
azacitidine or decitabine in an outpatient setting in patients with
acute myeloid leukemia. Hematological Oncology, 42(3), e3274. https://doi.org/10.1002/hon.3274 PMid:38711253
- Yassine,
F., Saad, F., Cheekati, M., Rutledge, J., Baksa, B., & Cherry, M.
(2024). Dose of Venetoclax in Combination with Hypomethylating Agents
and Outcomes of Patients with Acute Myeloid Leukemia: A Retrospective
Study. Blood, 144(Supplement 1), 2884. https://doi.org/10.1182/blood-2024-194499
- Qureshi,
Z., Altaf, F., Jamil, A., & Siddique, R. (2024). Safety, Efficacy,
and Predictive Factors of Venetoclax-Based Regimens in Elderly Acute
Myeloid Leukemia Patients: A Meta-Analysis. Clinical lymphoma, myeloma
& leukemia, 24(11), e835-e851. https://doi.org/10.1016/j.clml.2024.07.004 PMid:39218712
- Zhou,
Z., Tan, Y., Zhao, X., Suo, X., Bai, G., Bai, Y., Luo, Y., Lu, X.,
Yuan, L., Zhang, C., Li, Y., Gao, S., Zhang, J., Peng, H., Guo, P., Mi,
Y., & Liu, K. (2024). Venetoclax Combined with Three-Day Multi-
Frequency Decitabine (DEC3-VEN) VS Venetoclax Combined with Azacitidine
in Elderly Patients with Acute Myeloid Leukemia: a Phase III,
Prospective, Multicenter, Randomized Controlled Trial. Blood,
144(Supplement 1), 6035. https://doi.org/10.1182/blood-2024-202495
- Braish,
J., Montalban‐Bravo, G., Ravandi, F., Short, N. J., Kadia, T. M.,
Ohanian, M., Chien, K. S., Masárová, L., Sasaki, K., Yılmaz, M., Daver,
N., Borthakur, G., Kantarjian, H., & Garcia‐Manero, G. (2024).
Phase I/II Combination Study of Azacitidine and Venetoclax Post
Hypomethylating Agents Failure in High Risk MDS and CMML. Blood,
144(Supplement 1), 3209. https://doi.org/10.1182/blood-2024-208536
- Zhang,
L., Ge, R., Pan, D., Yue, P., Zhang, J., Bian, R., & Yan, X.
(2024). A real-world experience of venetoclax combined with
hypomethylating agents vs. monotherapy hypomethylating agents in
patients with myelodysplastic syndromes and chronic myelomonocytic
leukemia patients. Frontiers in pharmacology, 15, 1265840. https://doi.org/10.3389/fphar.2024.1265840 PMid:38756378 PMCid:PMC11096538
- Ginosyan,
A. A., Ashouri, K., Humayun, L., Ford, L., Baya, M., Hong, H.-J.,
Huang, E., Hom, B., Ashtiani, Y., Ann, B., Ireland, R. H., Mantri, S.
S., Greenlee, S., Siddiqi, I., Ali, A., Woan, K., Ladha, A., Tam, E.,
& Yaghmour, G. (2024). Optimizing Venetoclax Duration in
Combination with Hypomethylating Agents for Newly Diagnosed AML: Impact
on Treatment Response and Survival Outcomes. Blood, 144(Supplement 1),
2892. https://doi.org/10.1182/blood-2024-209246
- Tomlinson,
B., & De Lima, M. (2019). Allogeneic Hematopoietic Stem Cell
Transplantation for Acute Myeloid Leukemia (pp. 139-158). Elsevier. https://doi.org/10.1016/B978-0-323-56802-9.00009-2
- Ye,
Y., Chen, Y., Yu, G., Weng, G., Liu, Q., & Jin, H. (2023).
Comparison of two venetoclax-based regimens as salvage therapies
bridged to allogeneic hematopoietic cell transplantation in patients
with relapsed/refractory acute myeloid leukemia. Blood, 142(Suppl. 1),
7104. https://doi.org/10.1182/blood-2023-181616
- Lipof,
J. J., Loh, K. P., O'Dwyer, K. M., & Liesveld, J. L. (2018).
Allogeneic Hematopoietic Cell Transplantation for Older Adults with
Acute Myeloid Leukemia. Cancers, 10(6), 179. https://doi.org/10.3390/cancers10060179 PMid:29866998 PMCid:PMC6025016
- Ronnacker,
J., Urbahn, M.-A., Reicherts, C., Kolloch, L., Berning, P., Call, S.,
Floeth, M., Marx, J., Mikesch, J.-H., Schliemann, C., Lenz, G., &
Stelljes, M. (2024). Post-transplant relapse in patients transplanted
with active acute myeloid leukemia (AML) - Treatment options and
outcomes at a large AML referral center. Blood, 144(Suppl. 1), 2177. https://doi.org/10.1182/blood-2024-203502
- Göker, H., Çınar, O. E., Demiroğlu, H., Malkan, Ü. Y., Aladağ Karakulak, E., & Büyükaşık, Y. (2023). Venetoclax and Azacitidine Treatment in Relapsed Acute Myeloid Leukemia after Hematopoietic Stem Cell Transplantation: A Cohort Study in the Real-World Setting of a Tertiary Center. Turkish Journal of Haematology : official journal of Turkish Society of Haematology, 40(3), 213-215. https://doi.org/10.4274/tjh.galenos.2023.2023.0089 PMid:37314288 PMCid:PMC1047624
Supplementary Data
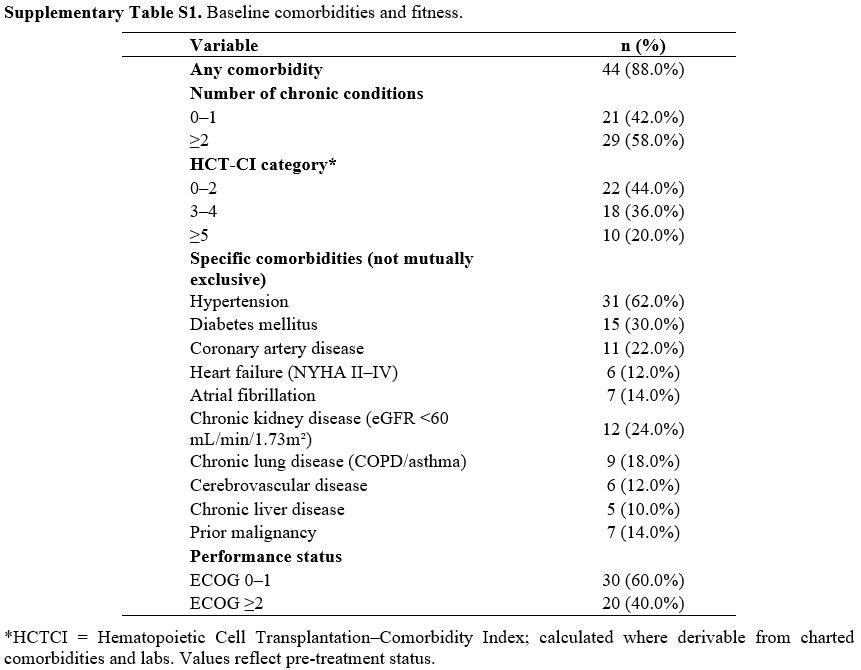 |
Supplementary Table S1. Baseline comorbidities and fitness. |
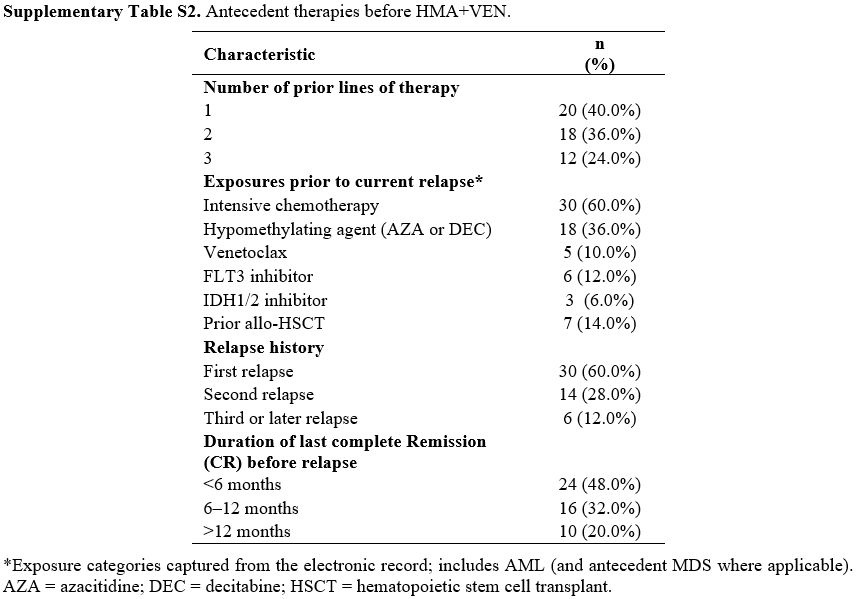 |
Supplementary Table S2. Antecedent therapies before HMA+VEN. |
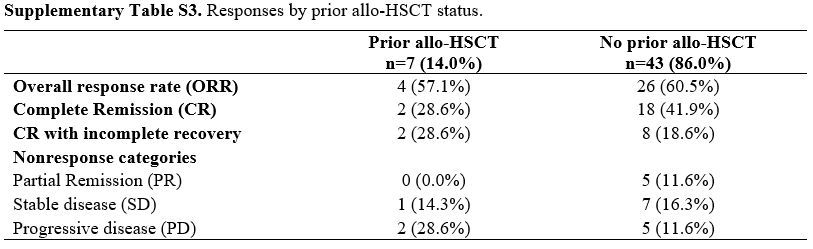 |
Supplementary Table S3. Responses by prior allo-HSCT status. |
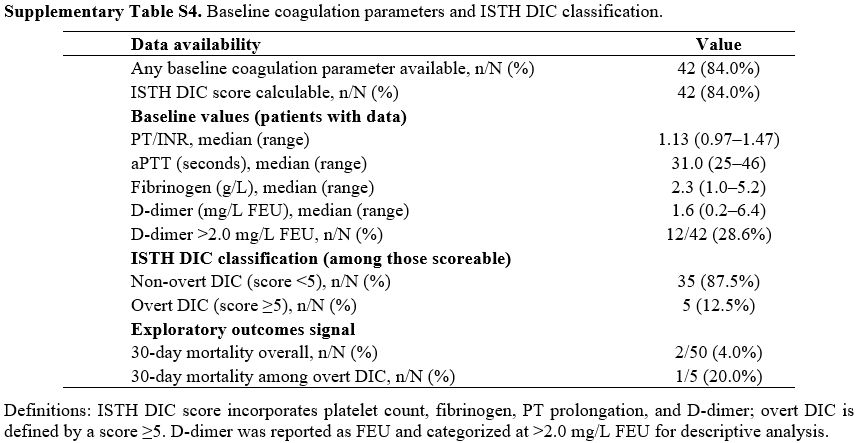 |
Supplementary Table S4. Baseline coagulation parameters and ISTH DIC classification. |