Biomarkers such as procalcitonin (PCT), C-reactive protein (CRP), and albumin have become pivotal in sepsis care, refining diagnosis, guiding therapy, and sharpening prognostic stratification.[6,7] PCT rises swiftly in bacterial infections and closely parallels disease severity,[8] whereas CRP - though less specific - offers high sensitivity for systemic inflammation.[9,10] Comparative studies consistently show PCT to be the more specific marker and CRP the more sensitive one.[10] Persistently elevated PCT or CRP levels, particularly in concert with leukocytosis, signal an increased risk of mortality.[11,12] Hypo-albuminemia further reflects capillary leak and poor nutritional reserves, adding complementary prognostic value. Integrating these biomarkers, therefore, enhances both the diagnostic precision and outcome prediction essential to effective sepsis management.
Growing evidence supports the integration of procalcitonin (PCT), C-reactive protein (CRP), and albumin into antibiotic-stewardship protocols, especially for respiratory infections and the critically ill. PCT has emerged as a pivotal biomarker, reliably guiding when to start and, crucially, when to stop antibiotics - thereby reducing unnecessary exposure and improving outcomes in sepsis and acute respiratory infections.[13,14] PCT-based algorithms can safely shorten treatment courses and lessen drug-related adverse effects.[14] CRP point-of-care testing similarly enhances decision-making in primary care: low levels help rule out serious infection and justify withholding antibiotics.[15,16] While both markers add value, PCT provides greater specificity but is less widely available and more expensive.[16] Albumin level, reflecting capillary leak and nutritional status, offers complementary prognostic insight. Embedding these biomarkers within stewardship programmes can therefore optimise patient care and curb antibiotic overuse.
This study will test whether tailoring antibiotics to biomarker levels - procalcitonin (PCT), C-reactive protein (CRP), and albumin - improves both clinical and economic outcomes in sepsis. Primary endpoints are total antibiotic-days, time to de-escalation, and the incidence of secondary infections. Secondary analyses will assess cost-effectiveness, including total hospital expenditure and 30-day readmission rates. By linking patient-centred outcomes with resource utilisation, the trial aims to show whether biomarker-guided therapy can simultaneously enhance care quality and curb unnecessary antibiotic use.
Participants and Methods
This study was conducted in Wujing Community Health Center, affiliated with Shanghai University of Traditional Chinese Medicine, to compare the efficacy of biomarker-guided antibiotic therapy against standard care in adult sepsis patients. The study was approved by the institutional review boards of Wujing Community Health Center, affiliated to Shanghai University of Traditional Chinese Medicine. All eligible patients underwent a rapid capacity assessment using the Aid‑to‑Capacity Evaluation. If the patient was deemed capable, written informed consent was obtained directly. If capacity was lacking, the attending physician sought written consent from the legally authorized representative (LAR). When neither the patient nor an LAR was available within 6 h of meeting inclusion criteria and delaying biomarker sampling would have compromised care, enrolment proceeded under the Institutional Review Board–approved emergency waiver of consent; written consent was subsequently obtained from the patient or LAR within 72 h. Participants (or their LARs) retained the right to withdraw at any time, and data were expunged should consent not be granted retrospectively.Inclusion criteria for the participants included: 1) 18 years or older; 2) diagnosed with sepsis according to the Sepsis-3 criteria within the past 24 hours; 3) admitted to the hospital through emergency departments or directly to intensive care units. Exclusion criteria were: 1) age under 18; 2) pregnancy; 3) documented anaphylaxis or severe IgE‑mediated hypersensitivity to all first‑line antibiotics, precluding their safe use. 4), presence of terminal illness where the life expectancy is less than one month or withdrawal from aggressive treatment is planned.
Patients were randomly assigned to the control group and the biomarker group, using a computer-generated sequence with stratification by site to ensure balanced allocation across different hospitals.
Sample‑size calculation and recruitment period. Power analysis (G*Power 3.1) indicated that, assuming a mean antibiotic duration of 11 days (SD 3) in standard care, 63 patients per group would provide 80% power (two‑sided α 0.05) to detect a 1.5‑day absolute reduction. Allowing for a 2% attrition rate, a target of 127 patients was set. Consecutive eligible adults were screened from January 1, 2023, to March 31, 2024; recruitment stopped automatically when the target was attained and all participants had completed a 28‑day follow‑up. No interim efficacy or futility analyses were conducted.
Interventions. Empiric therapy followed the Wujing Community Health Center 2023 Sepsis Guideline: piperacillin–tazobactam or cefoperazone–sulbactam (community‑onset); meropenem or imipenem–cilastatin (health‑care‑associated or MDR risk); addition of vancomycin or linezolid when MRSA was suspected. The specific agent, dose, and de‑escalation target were chosen by the attending physician in consultation with the stewardship team. The intervention under study was limited to timing decisions based on PCT/CRP/albumin trajectories; no investigational antibiotic was administered.
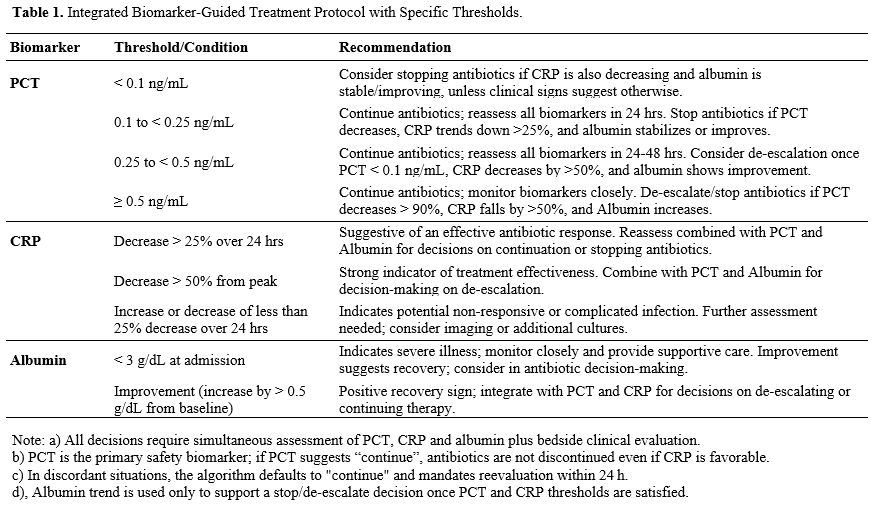
Biomarker-Guided Therapy Group: Antibiotic therapy adjustments were based on predefined thresholds of PCT, CRP, and Albumin as outlined in Table 1. Decisions to escalate, de-escalate, or discontinue antibiotics were made in conjunction with clinical assessment, including signs of infection improvement or deterioration.
Control Group: Participants received antibiotics based on the current best practice guidelines without biomarker integration. Therapy adjustments were made on clinical judgment without specific biomarker targets.
Clinical decision algorithm: At each daily stewardship round, the attending physician reviewed PCT, CRP, and albumin together and applied the following hierarchy: (1) primary trigger = PCT; (2) secondary modifier = CRP trend; (3) supportive modifier = albumin trend. Antibiotic treatment was discontinued when all of these apply: PCT < 0.10 ng/mL AND CRP has fallen by ≥ 50% from its peak AND albumin has stabilized or risen (≥ baseline). Continue current therapy when PCT is 0.10-0.49 ng/mL or PCT is falling but either CRP has fallen < 50% or albumin remains < baseline; reassess in 24 h. Escalate or broaden cover when PCT ≥ 0.50 ng/mL or PCT has risen by > 25% from the previous value, irrespective of CRP/albumin, unless a non‑infectious cause is evident. If PCT and CRP were discordant, the physician prioritized the less favorable marker to maximize safety. Albumin changes were never used in isolation to stop antibiotics.
Microbiological work‑up and antibiotic strategy. Blood cultures (two sets from separate venipunctures) plus site‑specific cultures (sputum, urine, abdominal fluid) were obtained before the first antibiotic dose wherever feasible. Empiric therapy followed local sepsis guidelines. If a pathogen and susceptibility profile became available within 72 h, therapy was narrowed to the most appropriate agent; this was classified as culture‑targeted. Cases in which no pathogen grew, or therapy remained broad‑spectrum despite a pathogen, were categorized as empiric‑only.
Outcome Measures. Primary Outcomes: 1) duration of antibiotic therapy, defined as the number of days from initiation to cessation of antibiotic treatment. 2), 28-day all-cause mortality. 3), Hospital length of stay from admission to discharge.
Secondary Outcomes: 1) 30-day hospital readmission rates. 2) Incidence of secondary infections during the hospital stay. 3) Economic evaluation, including direct hospital costs associated with treatment.
Data Collection. Timing of Measurements: 1) Biomarkers were measured at admission (baseline), and then at predefined intervals of 24, 48, and 72 hours, and subsequently based on clinical indications. 2) Clinical assessments were conducted daily by attending healthcare professionals.
Statistical Analysis. The results of numerical data were presented as mean ± standard deviation (SD), while the categorical data were presented as numbers and percentages. Continuous variables were compared using Student's t-test or Mann-Whitney U test, depending on data distribution. Categorical variables were analyzed using the chi-square test or Fisher's exact test as appropriate. Time-to-event outcomes were analyzed using the Kaplan-Meier method and compared using the log-rank test.
Results
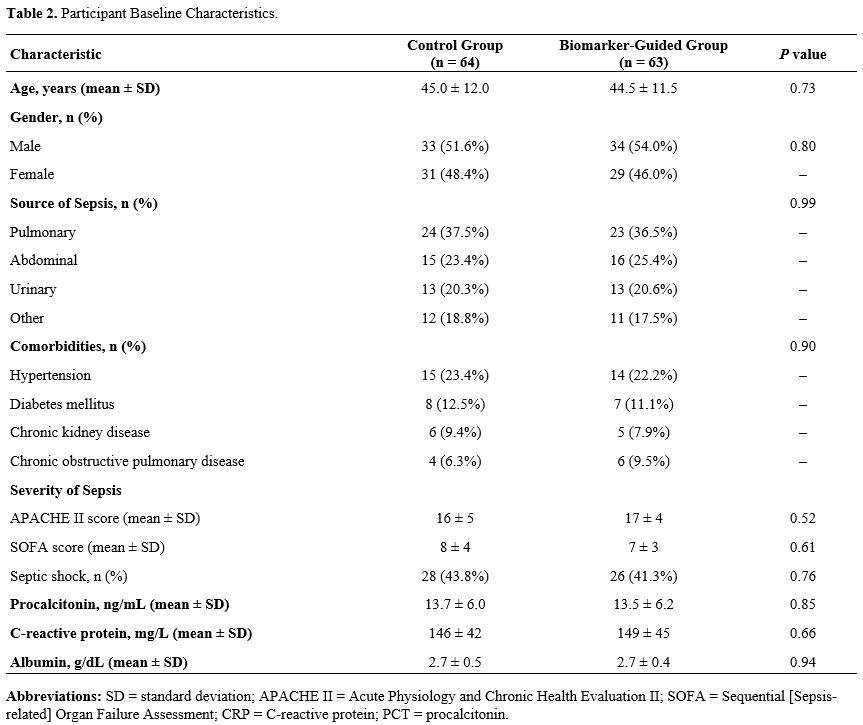
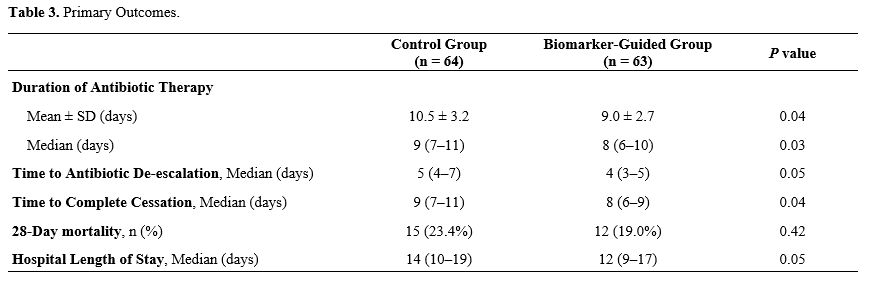
Participant Baseline Characteristics. A total of 127 patients were enrolled, with 64 assigned to standard care and 63 to biomarker-guided therapy (Table 2). Baseline demographics and clinical characteristics - including age, sex, infection source, and comorbidity profile - were comparable between the two groups, with no statistically significant differences. Mean age was comparable between groups (45.0 ± 12.0 years in the control arm vs 44.5 ± 11.5 years in the biomarker-guided arm; P = 0.73). Sex distribution was similarly balanced, with men representing 51.6% of the control group and 54.0% of the biomarker-guided group (P = 0.80). The primary sources of sepsis - pulmonary, abdominal, urinary, and others - were also evenly distributed between the groups (P = 0.99). The prevalence of major comorbidities - including hypertension, diabetes mellitus, chronic kidney disease, and chronic obstructive pulmonary disease - did not differ significantly between groups (P = 0.90). The severity of sepsis, assessed by APACHE II and SOFA scores, and the incidence of septic shock were comparable.Impact of Biomarker-Guided Antibiotic Therapy on Treatment Outcomes in Sepsis Patients. Antibiotic stewardship improved markedly under biomarker guidance. Mean treatment duration fell to 9.0 ± 2.7 days versus 10.5 ± 3.2 days with standard care (P = 0.04; Table 3). Median course length likewise declined - from 9 days (IQR 7–11) in the control arm to 8 days (IQR 6–10) in the biomarker-guided arm (P = 0.03). Median time to antibiotic de-escalation dropped to 4 days (IQR 3–5) in the biomarker-guided group versus 5 days (IQR 4–7) with standard care (P = 0.05). Median time to complete cessation likewise shortened from 9 days (IQR 7–11) to 8 days (IQR 6–9) (P = 0.04).. However, there was no significant difference in 28-day mortality rates between the groups, with 19.0% in the biomarker-guided group versus 23.4% in the control group (P = 0.42) The median hospital length of stay was slightly shorter in the biomarker-guided group, 12 days (range 9–17), compared to 14 days (range 10–19) for the control group (P = 0.05).
Microbiological findings and antibiotic strategy. Blood cultures were positive in 24/64 control patients (37.5%) and 23/63 biomarker‑guided patients (36.5%) (P = 0.92). Conversion from empiric to culture‑targeted therapy within 72 h occurred in 18 (28.1%) vs 17 (27.0%) patients, respectively (P = 0.93). Adjusting the primary outcome for culture status and antibiotic strategy left the treatment effect essentially unchanged (β = ‑1.4 days, 95% CI ‑2.3 to ‑0.5, P = 0.003; Supplementary Table S1).
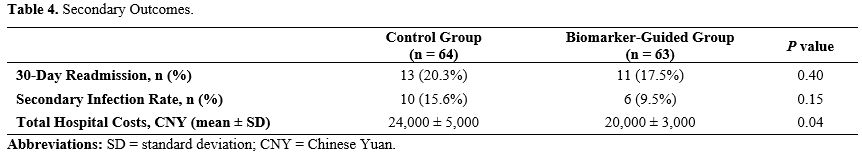
Economic and Clinical Efficiency of Biomarker-Guided Therapy in Sepsis Management. In evaluating secondary outcomes for sepsis management between a control group and a biomarker-guided group, there were important distinctions in hospital costs and infection rate (Table 4).
The 30-day readmission rates were similar between groups, with 20.3% in the control group and 17.5% in the biomarker-guided group (P = 0.40). Secondary infection rates were slight lower in the biomarker-guided group at 9.5% compared to 15.6% in the control group (P = 0.15). Notably, there was a significant reduction in total hospital costs for the biomarker-guided group (P = 0.04)
Discussion
This study confirms that biomarker-guided antibiotic therapy - using procalcitonin, C-reactive protein, and albumin thresholds - shortens treatment duration without sacrificing clinical efficacy. By tailoring antimicrobial decisions to individual inflammatory profiles, we add persuasive evidence for a personalized approach to sepsis care. As health systems pivot toward precision medicine, integrating biomarker guidance could curb unnecessary antibiotic exposure, mitigate resistance, and ultimately ease the global burden of sepsis.The antibiotic savings we observed mirror and reinforce the broader stewardship literature. Stopping therapy when PCT falls below prespecified thresholds can trim exposure by up to 25% without increasing mortality.[17] The ADAPT-Sepsis trial likewise showed that PCT-guided protocols shortened courses in hospitalized patients while maintaining safety.[18] Subsequent studies in sepsis and lower-respiratory-tract infections have confirmed that PCT algorithms safely reduce antimicrobial use, improving patient safety and cutting costs.[13] A recent systematic review reached the same conclusion, reporting significant reductions in antibiotic days with no compromise in outcomes.[14] CRP point-of-care testing has also proved valuable in primary care, reliably distinguishing bacterial from viral infections and steering prescribers toward more judicious antibiotic use.[15]
Consistent with growing evidence favoring personalized antimicrobial strategies,[14] the biomarker-guided cohort required a shorter mean antibiotic course. Curtailing exposure not only optimizes individual outcomes but also helps prevent secondary infections and slows the emergence of antimicrobial resistance (AMR).[16] Integrating biomarkers into sepsis protocols thus dovetails with global efforts toward judicious antibiotic use. This alignment is increasingly critical as multidrug-resistant pathogens proliferate, making innovative, biomarker-guided stewardship essential for safeguarding the efficacy of existing treatments.[19]
Although our cohort achieved shorter hospital stays and reduced antibiotic exposure, we did not detect a statistically significant decline in secondary infections. This finding diverges from reports that credit biomarker-guided protocols with lowering secondary-infection rates through more precise antimicrobial targeting.[20] Several factors may explain the discrepancy. First, methodological variations - such as a smaller sample size and a single-center design - limit statistical power and external validity. Second, patient heterogeneity (e.g., differing baseline risk for nosocomial infection) can dilute effect estimates. Finally, our use of fixed biomarker cut-offs may blunt responsiveness; studies that employ dynamic, patient-specific thresholds have documented superior outcomes over static algorithms.[21] Future work should therefore adopt adaptive thresholds and enroll larger, multicenter populations to clarify the true impact of biomarker guidance on secondary infections.
Biomarker-guided therapy improves both clinical outcomes and economic efficiency. By curbing unnecessary antibiotic exposure, it lowers the risk of secondary infections and drug-related adverse events. In our cohort, this approach shortened antibiotic courses and reduced length of stay - findings that echo prior meta-analyses.[22] Real-time biomarker trends also let clinicians tailor treatment duration precisely, a strategy shown to enhance antimicrobial stewardship and cut direct hospital costs.[23]
Despite the encouraging findings, this study has important limitations. Foremost, the sample size of 127 patients - adequate for an exploratory analysis - restricts statistical power for secondary endpoints such as secondary-infection rates and longer-term outcomes. Although the trial was adequately powered for its primary endpoint, the sample size was insufficient to robustly analyze secondary outcomes such as infection relapse and long‑term morbidity. The study’s mostly homogeneous sample - adult patients treated within a single healthcare system - limits the applicability of our findings to other groups, such as pediatric or elderly populations and those in low-resource settings. Systematic reviews consistently highlight the need for larger, more diverse cohorts to validate biomarker thresholds across different demographic and clinical contexts.[24] Future research should therefore prioritize multicenter trials that enroll heterogeneous patient populations and incorporate longer follow-up. Region-specific calibration of biomarker cut-offs, along with continued innovation in affordable point-of-care diagnostics, will be vital to make biomarker-guided protocols broadly feasible and clinically relevant worldwide.
Conclusions
This study highlights the clear advantages of biomarker-guided antibiotic therapy for sepsis. By incorporating real-time procalcitonin, C-reactive protein, and albumin measurements, we show that antibiotics can be tailored more precisely—shortening treatment courses, lowering hospital costs, and preserving patient safety. These gains, achieved without compromising clinical outcomes, point to a practical route for curbing antibiotic overuse and resistance. Our findings, therefore, strengthen the case for integrating biomarker-based algorithms into routine sepsis care and lay a solid foundation for future work — especially studies that probe long-term outcomes and refine biomarker thresholds for diverse patient populations.Ethical Approval
The study was approved by the institutional review boards of Wujing Community Health Center, affiliated to Shanghai University of Traditional Chinese Medicine. Written informed consent was obtained from all participants, in accordance with the Declaration of Helsinki.Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.Author Contribution Statement
The authors confirm contribution to the paper as follows: study conception and design: Y.S.; data collection: J.C.; analysis and interpretation of results: J.C.; draft manuscript preparation: J.C., Y.S. All authors reviewed the results and approved the final version of the manuscript.References
- Watson RS, Carrol ED, Carter MJ, Kissoon N, Ranjit
S, Schlapbach LJ. The burden and contemporary epidemiology of sepsis in
children. Lancet Child Adolesc Health. 2024;8(9):670-681.
doi:10.1016/S2352-4642(24)00140-8 https://doi.org/10.1016/S2352-4642(24)00140-8 PMid:39142741
- Hedjal
J. Chapter 3 - The Epidemiology of Sepsis. In: Borges M, Hidalgo J,
Perez-Fernandez J, eds. The Sepsis Codex. Elsevier; 2023:11-15.
doi:10.1016/B978-0-323-88271-2.00027-4. https://doi.org/10.1016/B978-0-323-88271-2.00027-4
- Plaja A, Tobías D. Código sepsis. Actualización en Medicina de Familia. 2024;483-486. doi:10.55783/AMF.200805. https://doi.org/10.55783/AMF.200805
- Garvey
M. Hospital Acquired Sepsis, Disease Prevalence, and Recent Advances in
Sepsis Mitigation. Pathogens. 2024;13(6):461. Published 2024 May 30.
doi:10.3390/pathogens13060461 https://doi.org/10.3390/pathogens13060461 PMid:38921759 PMCid:PMC11206921
- Aljefri
A, Almutairi L, Alraddadi M, et al. Definition, epidemiology and
characterization of sepsis. Int J Community Med Public Health. 2023;11.
doi:10.18203/2394-6040.ijcmph20233852. https://doi.org/10.18203/2394-6040.ijcmph20233852
- Sireesha,
Vankodoth F, Fatima F, Sultana S, Kumar MSS, Pravarsha Y, Tatikonda RR.
A Comprehensive Review on Biomarker and Its Role in Diseases. Cardiol
Angiol Int J. 2024;13(1):75-81. doi:10.9734/ca/2024/v13i1395. https://doi.org/10.9734/ca/2024/v13i1395
- Mishra D, Mahajan D. BIOMARKERS. 2024. doi:10.58532/V3BFBT3P1CH5. https://doi.org/10.58532/V3BFBT3P1CH5 PMid:38248295
- Tyagi
N, Gawhale S, Patil MG, Tambolkar S, Salunkhe S, Mane SV. Comparative
Analysis of C-reactive Protein and Procalcitonin as Biomarkers for
Prognostic Assessment in Pediatric Sepsis. Cureus. 2024;16(7):e65427.
Published 2024 Jul 26. doi:10.7759/cureus.65427 https://doi.org/10.7759/cureus.65427
- Daud
M, Khan MB, Qudrat QU, et al. Role of C-reactive Protein and
Procalcitonin in Early Diagnostic Accuracy and Their Prognostic
Significance in Sepsis. Cureus. 2024;16(9):e70358. Published 2024 Sep
27. doi:10.7759/cureus.70358 https://doi.org/10.7759/cureus.70358
- Hossain
Natasha S, Ahsan ASMA, Fatema K, Ahmed F, Sultana R. Procalcitonin and
C-Reactive Protein in Critically Ill Patients with Sepsis and Septic
Shock Admitted in an ICU of a Tertiary Care Hospital in Bangladesh.
Bangladesh Crit Care J. 2024;12(1):4-10. doi:10.3329/bccj.v12i1.72419. https://doi.org/10.3329/bccj.v12i1.72419
- Doganci
M, Eraslan Doganay G, Sazak H, et al. The Utility of C-Reactive
Protein, Procalcitonin, and Leukocyte Values in Predicting the
Prognosis of Patients with Pneumosepsis and Septic Shock. Medicina
(Kaunas). 2024;60(10):1560. Published 2024 Sep 24.
doi:10.3390/medicina60101560 https://doi.org/10.3390/medicina60101560 PMid:39459346 PMCid:PMC11509754
- Melek
D, Güler ED, Hilal S, et al. The Utility of C-Reactive Protein,
Procalcitonin, and Leukocyte Values in Predicting the Prognosis of
Patients with Pneumosepsis and Septic Shock. 2024.
doi:10.20944/preprints202408.1007.v1. https://doi.org/10.20944/preprints202408.1007.v1
- Kyriazopoulou
E, Giamarellos-Bourboulis EJ. Antimicrobial Stewardship Using
Biomarkers: Accumulating Evidence for the Critically Ill. Antibiotics
(Basel). 2022;11(3):367. Published 2022 Mar 9.
doi:10.3390/antibiotics11030367 https://doi.org/10.3390/antibiotics11030367 PMid:35326830 PMCid:PMC8944654
- Schuetz
P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided
antibiotic stewardship: an international experts consensus on optimized
clinical use. Clin Chem Lab Med. 2019;57(9):1308-1318.
doi:10.1515/cclm-2018-1181 https://doi.org/10.1515/cclm-2018-1181 PMid:30721141
- Staiano
A, Bjerrum L, Llor C, et al. C-reactive protein point-of-care testing
and complementary strategies to improve antibiotic stewardship in
children with acute respiratory infections in primary care. Front
Pediatr. 2023;11:1221007. Published 2023 Oct 12.
doi:10.3389/fped.2023.1221007 https://doi.org/10.3389/fped.2023.1221007 PMid:37900677 PMCid:PMC10602801
- Vingerhoets
S, Mueller Y, Pedrazzini B, Boillat-Blanco N, Lhopitallier L.
Prescription des antibiotiques guidée par les biomarqueurs en médecine
de famille : CRP ou PCT ? [Biomarker-guided antibiotics prescription
for lower respiratory tract infections in primary care : CRP or PCT ?].
Rev Med Suisse. 2022;18(781):948-952.
doi:10.53738/REVMED.2022.18.781.948 https://doi.org/10.53738/REVMED.2022.18.781.948 PMid:35543687
- Ling
RR, Somani J, Ramanathan K. Biomarker-Guided Antibiotic Discontinuation
in Adults Critically Ill With Sepsis: Harnessing Network Meta-Analysis
to Guide Clinical Therapy. Crit Care Med. 2024;52(10):1658-1660.
doi:10.1097/CCM.0000000000006381. https://doi.org/10.1097/CCM.0000000000006381 PMid:39283212
- Dark
P, Perkins GD, McMullan R, et al. biomArker-guided Duration of
Antibiotic treatment in hospitalised Patients with suspecTed Sepsis
(ADAPT-Sepsis): A protocol for a multicentre randomised controlled
trial. J Intensive Care Soc. 2023;24(4):427-434.
doi:10.1177/17511437231169193. https://doi.org/10.1177/17511437231169193 PMid:37841304 PMCid:PMC10572477
- Alaoui
Mdarhri H, Benmessaoud R, Yacoubi H, Seffar L, Guennouni Assimi H,
Hamam M, Boussettine R, Filali-Ansari N, Lahlou FA, Diawara I, et al.
Alternatives Therapeutic Approaches to Conventional Antibiotics:
Advantages, Limitations and Potential Application in Medicine.
Antibiotics. 2022; 11(12):1826. https://doi.org/10.3390/antibiotics11121826 PMid:36551487 PMCid:PMC9774722
- Llitjos
JF, Carrol ED, Osuchowski MF, et al. Enhancing sepsis biomarker
development: key considerations from public and private perspectives.
Crit Care. 2024;28(1):238. Published 2024 Jul 13.
doi:10.1186/s13054-024-05032-9. https://doi.org/10.1186/s13054-024-05032-9 PMid:39003476 PMCid:PMC11246589
- Póvoa
P, Coelho L, Dal-Pizzol F, et al. How to use biomarkers of infection or
sepsis at the bedside: guide to clinicians. Intensive Care Med.
2023;49:142-153. doi:10.1007/s00134-022-06956-y. https://doi.org/10.1007/s00134-022-06956-y PMid:36592205 PMCid:PMC9807102
- Trapnell
BC. A novel biomarker-guided immunomodulatory approach for the therapy
of sepsis. Am J Respir Crit Care Med. 2009;180(7):585-586.
doi:10.1164/rccm.200907-1095ED. https://doi.org/10.1164/rccm.200907-1095ED PMid:19762591
- Aulin
LBS, de Lange DW, Saleh MAA, van der Graaf PH, Völler S, van Hasselt
JGC. Biomarker-Guided Individualization of Antibiotic Therapy. Clin
Pharmacol Ther. 2021;110(2):346-360. doi:10.1002/cpt.2194. https://doi.org/10.1002/cpt.2194 PMid:33559152 PMCid:PMC8359228
- Nora D, Salluh J, Martin-Loeches I, Póvoa P. Biomarker-guided antibiotic therapy-strengths and limitations. Ann Transl Med. 2017;5(10):208. doi:10.21037/atm.2017.04.04. https://doi.org/10.21037/atm.2017.04.04 PMid:28603723 PMCid:PMC5451622