The POLARIX trial indicated that polatuzumab vedotin (Pola) combined with rituximab, cyclophosphamide, doxorubicin, and prednisolone (PRCHP) resulted in a lower risk of disease progression and relapse than R-CHOP in patients aged <80 years.[7] We previously reported on 38 patients aged over 80 years with DLBCL who underwent PRCHP in a clinical setting, and conducted a retrospective observational study to evaluate its efficacy and safety.[8] PRCHP and RCHOP protocols for frail patients aged >75 years or >80 years, which expanded the POLARIX trial, are being advanced by the Polar-BEAR trial in an open-label, randomized Nordic Lymphoma Group Phase III trial.[9] In the POLASTAR trial, the best response at end of treatment (55 patients over 80 years old) was reported as ORR 80.0%, (67.6-88.4) CRR 87.3% (76.0-93.7) at the real world date, in which 60 patients over 80 years old were included, indicating that a high response rate may be expected even in elderly patients.[10] However, to date, no study has compared the mini-R-CHOP and mini-PRCHP in patients aged 80 years or older. Herein, we report the results of a retrospective comparative study evaluating the outcomes of frontline dose-attenuated PRCHP versus dose-attenuated R-CHOP administered to patients with DLBCL.
Materials and Methods
This single-center retrospective study was conducted between January 2018 and December 2024. All patients received at least one cycle of chemotherapy, including those who discontinued treatment early due to toxicity or disease progression; their relative dose intensity (RDI) was also monitored. The inclusion criteria were age >80 years, pathologically confirmed diagnosis of DLBCL according to the World Health Organization classification (2022 version),[11] and not having received either first-line PRCHP or RCHOP. Specific diagnostic criteria for DLBCL include pathologically confirmed diffuse infiltration of large, atypical lymphocytes that are CD20-positive, CD3-negative, and demonstrate Ki-67 staining of> 60%. The exclusion criteria were previous radical irradiation before treatment for DLBCL, CNS involvement at initial presentation, and high-grade B-cell lymphoma with MYC and BCL2 rearrangement. The R-CHOP regimen consisted of prednisolone (100 mg/day orally on days 1–5), doxorubicin (50 mg/m² intravenously on day 1), cyclophosphamide (750 mg/m² intravenously on day 1), vincristine (1.4 mg/m² intravenously on day 1), and rituximab (375 mg/m² intravenously on day 1), administered every 21 days for six cycles. The PRCHP regimen consisted of prednisolone (100 mg/day orally on days 1–5), doxorubicin (50 mg/m² intravenously on day 1), cyclophosphamide (750 mg/m² intravenously on day 1), polatuzumab vedotin (1.8 mg/m² intravenously on day 1), and rituximab (375 mg/m² intravenously on day 1), administered every 21 days for six cycles. Dose adjustments were made at the discretion of the attending physicians. The terms “dose-attenuated” PRCHP and RCHOP refer to reduced regimens individualized at the discretion of the treating physician, based on the patient's age, frailty, and comorbidities, rather than those defined in the protocol used in the POLARIX or Peyrade trials.[5] Herein, typical modifications included reducing the doses of cyclophosphamide and doxorubicin to 50–75% of standard doses, and limiting vincristine (in RCHOP) to a maximum of 1.0 mg or omitting it entirely. Polatuzumab vedotin was generally administered at full dose unless adverse events occurred. Prednisolone was maintained at 40-60mg/day, depending on tolerability. These dose adjustments were consistent with real-world practice for super-aged populations. The RDI was calculated based on a planned treatment cycle of 21 days. Dose intensity (DI) was defined as the scheduled dose per cycle (mg/m²) divided by the planned duration of the cycle (in weeks). The RDI (%) was determined by dividing the actual dose intensity by the target dose intensity and multiplying the result by 100. The total average relative dose intensity (tARDI) represented the RDI delivered by each chemotherapeutic agent (doxorubicin and cyclophosphamide) across all treatment cycles. It was calculated by dividing the total actual dose (mg/m²) over the total treatment duration (weeks) by the total planned dose (mg/m²) over the planned treatment duration. In cases with fewer than six cycles due to disease progression or death, the number of cycles of regimens administered without any reduction or delay was regarded as the maximum ARDI value of 100%.Older patients differ in characteristics such as frailty, underlying conditions, and nutritional status, compared with younger patients. Therefore, frailty was assessed based on the frailty score obtained from the retrospective analysis. In accordance with the simplified frailty score (SFS), a geriatric assessment was performed before administering PRCHP therapy using three tools: ADL, Charlson Comorbidity Index, Geriatric Nutritional Risk Index (GNRI), and age <85 years.[12]
A portion of the present sample (n = 38) overlaps with that described in our previous report, which focused on the real-world outcomes of Pola-R-CHP without adjusting for frailty.[8] However, the current study expands on that work by incorporating a propensity score–matched comparison (PSM) between PRCHP and RCHOP, stratified by frailty, and includes a longer follow-up period with more comprehensive outcome measures.
This study adhered to the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Shonan Kamakura General Hospital on Jun 4, 2024 (approval number TGE2422-024).
Propensity score matching. Before the analysis, PSM was used to reduce selection bias between the PRCHP and RCHOP groups. A logistic regression model was used to calculate the propensity scores. The variables included in the model were Ann Arbor stage, International Prognostic Index, and SFS. Patients in the two groups were matched 1:1 using nearest-neighbor matching based on propensity scores. A caliper width of 0.2 was used. After matching, standardized differences of <0.1 for all covariates indicated a balanced patient cohort for further analysis. Unmatched patients were excluded from this study.
Study outcomes. The primary endpoint of the study was 12-month progression-free survival (PFS) as defined by the Lugano 2014 criteria.[13] Secondary endpoints included overall response rate (ORR), complete response rate (CRR), overall survival (OS), and adverse event (AE) profile. PFS was defined as the time from the initiation of the first-line treatment to the earliest date of documented progression, relapse, or death from any cause. OS was measured from the start of treatment until death from any cause. The ORR and CRR were evaluated as complications of frontline therapy according to the Lugano 2014 criteria. Both PFS and OS were censored at the last follow-up of the living patients. AEs were evaluated both before and after propensity score matching. OS was measured from the date of inclusion to the date of death, regardless of the cause.
To address differences in follow-up durations between treatment groups, a landmark analysis was conducted on day 120. This time point was selected because both PRCHP and RCHOP regimens are typically administered every 21 days for six cycles, resulting in a total planned treatment duration of about 120 days. Therefore, patients who survived without progression for at least 120 days were included in this analysis to evaluate post-treatment outcomes in a balanced cohort.
Statistical analysis. We employed the Kaplan–Meier method to estimate OS and PFS rates. The p-value was computed using the log-rank test, with p < 0.05 considered statistically significant. We used Cox regression analysis to calculate the odds ratios (OR) in both univariate and multivariate contexts. All analyses were conducted using EZR[14] (Saitama Medical Center, Jichi Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files.statemedEN.html; Kanda, 2012).
To address potential temporal bias due to the difference in treatment approval dates, the diagnosis year (before vs. after August 2022) was included as a covariate in the multivariate Cox proportional hazards model. An interaction term between treatment group and diagnosis year was also tested, but could not be estimated due to the absence of patients treated with Pola-R-CHP before 2022.
Results
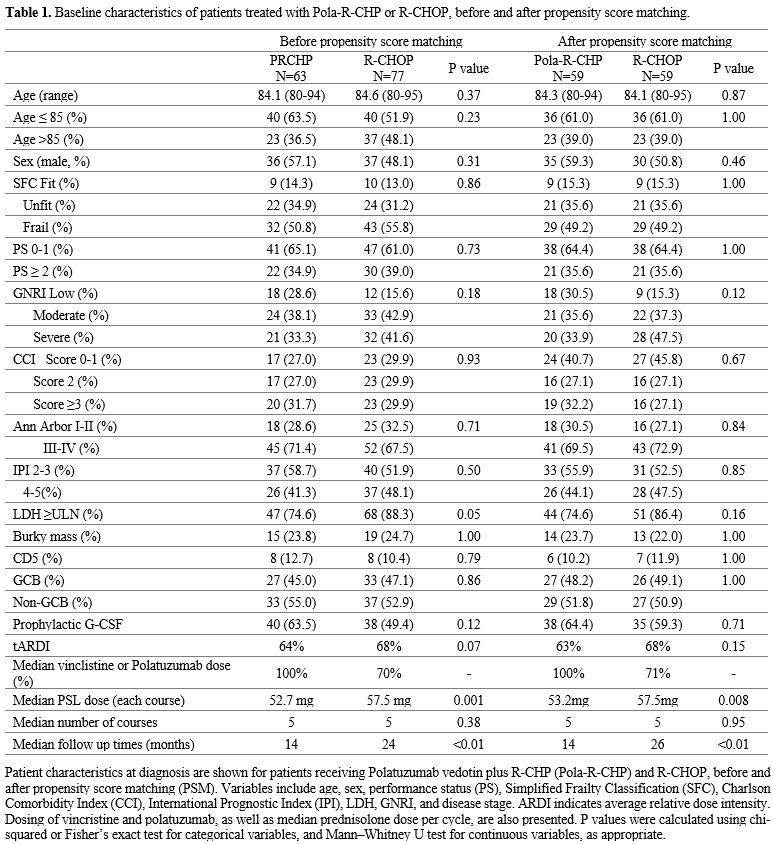
A total of 76 patients with newly diagnosed DLBCL were treated with RCHOP, and 63 patients were treated with PRCHP at our institute between January 2016 and December 2024. The baseline characteristics were compared (Table 1). Before PSM, the RCHOP group had more patients aged ≥85 years, PS ≥2, SFS frail group, and GNRI severe risk (>85 years old: RCHOP 48%, PolaRCHP 36%, p=0.23, PS ≥2: RCHOP 39%, PolaRCHP 34%, SFS frail: RCHOP 55%, PolaRCHP 50%, p=0.86, GNRI severe: RCHOP 41%, PolaRCHP 33%, p=0.18), and tARDI and PSL dose tended to be higher in RCHOP (tARDI: RCHOP 68%, PolaRCHP 64%, p=0.07, median PSL dose in each cycles; RCHOP 57.5 mg, PRCHP 52.7 mg, p=0.001) (Table 1). The median doses for doxorubicin and cyclophosphamide were 35.5 mg/m² and 532 mg/m² in the PRCHP group, and 33.5 mg/m² and 503 mg/m² in the RCHOP group, respectively. Pola is a CD79b-directed antibody–drug conjugate that did not require dose reduction at treatment initiation or due to peripheral neuropathy (PN) during the treatment course, whereas vincristine (VCR) was administered at a reduced dose in all cases.PSM was performed to balance covariates between the PRCHP and RCHOP groups. This resulted in 59 well-balanced patient pairs in the PRCHP and RCHOP groups for further survival and prognostic analyses (Table 1). The median age in each group was 84 years (p = 0.88); the proportion of individuals aged 85 years or older was similar in both groups at 39% (p = 1.00). Patients were classified as fit (n= 9, 15%), unfit (n=21, 36%), or frail (n=29, 49%) according to their frailty scores within each group (p=1.00). The median tARDI was 63% in PRCHP and 68% in RCHOP (p = 0.15), and the median number of courses was similar in both groups, at five courses (p =0.95) (Table 1). At the median follow-up, PRCHP was administered at 14 months and RCHOP at 24 months (p<0.01).
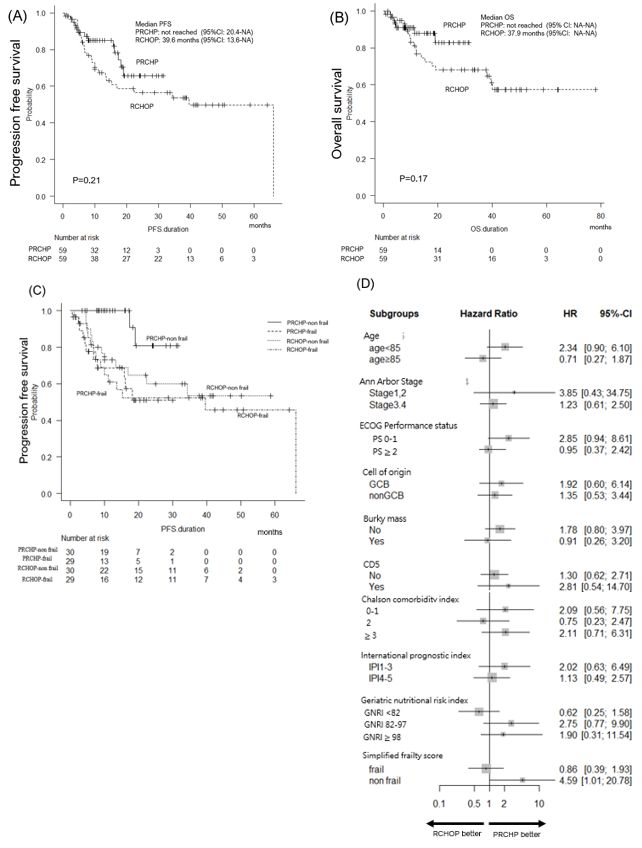
The 12-month PFS rates were 85.1% and 67.3% in patients treated with PRCHP and RCHOP, respectively (p=0.21; hazard ratio [HR]: 1.43; 95% CI: 0.72–2.74) (Figure 1A). The 12-month OS rate was 88.0% and 78.9% for PRCHP and RCHOP, respectively (p=0.18; HR: 1.85; 95% CI: 0.76-4.52; Figure 1B), with no difference in PFS and OS between those restricted to those aged> 80 years. In addition, the ORR was 94.9% and 98.3% (p=0.62), the CRR was 81.4% and 74.6% for PRCHP and RCHOP, respectively (p=0.28), and the best response rates were also similar. As shown in Figures 1C and D, the benefit of prolonged PFS was obtained in SFS non-frail (fit and unfit) cases. In the frail group, the 12-month PFS showed no differences (PRCHP 68.7% vs. RCHOP 61.9%, HR; 0.83, 95% CI: 0.39–1.93, p=0.87); whereas, in the non-frail (fit and unfit) patients, the PFS was significantly higher for patients in the PRCHP group than for those in the RCHOP group (PRCHP 100% vs. RCHOP 73.0%, HR; 4.59, 95% CI: 1.01–20.8, p=0.04). In a multivariate Cox model including age, IPI, and treatment era (before vs. after 2022), the HR for Pola-R-CHP vs. R-CHOP was 0.5865 (95% CI: 0.1679–2.048; p = 0.403), while diagnosis before 2022 showed a non-significant association with worse PFS (HR=2.757; 95% CI: 0.820–9.269; p=0.101). The interaction term between the treatment group and treatment year could not be estimated due to the lack of Pola-R-CHP cases prior to 2022.
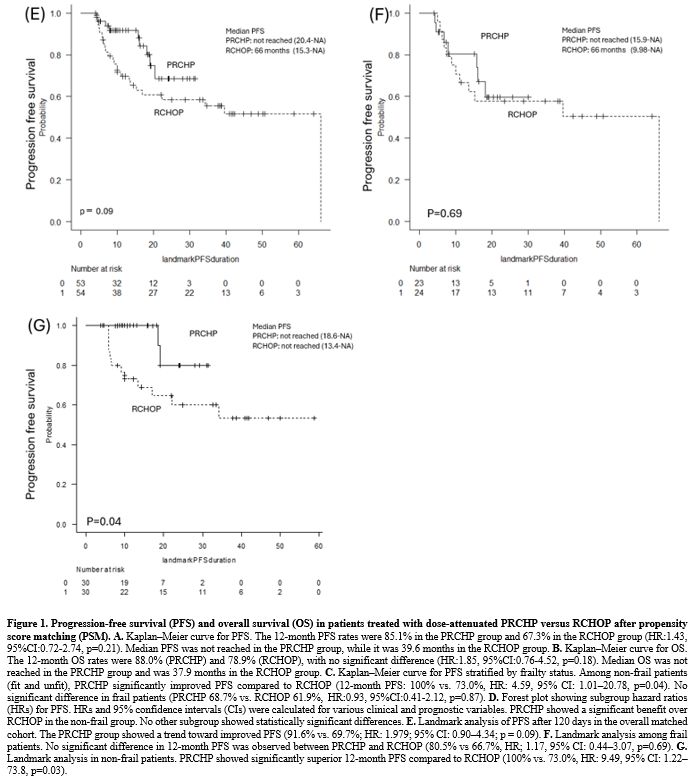
In a landmark analysis using a 120-day cutoff to reduce bias from differences in follow-up time and early events, the 12-month PFS was 91.6% in the PRCHP group and 69.7% in the RCHOP group (HR: 1.979; 95% CI: 0.90–4.34; p=0.09) (Figure 1E). Among frail patients, there was no significant difference in 12-month PFS (PRCHP: 80.5% vs. RCHOP: 66.7%; HR: 1.17; 95% CI: 0.44–3.07; p = 0.69) (Figure 1F); however, in non-frail patients, the PRCHP group showed a superior 12-month PFS compared to the RCHOP group (100% vs. 73.0%; HR: 9.49; 95% CI: 1.22–73.8; p=0.03) (Figure 1G).
 |
In addition, to assess the robustness of these findings, a sensitivity analysis using multivariate Cox regression was performed in the entire unmatched cohort (Table 2). This model included age, sex, simplified frailty score, performance status, IPI, and other relevant covariates. The treatment regimen was not independently associated with PFS (HR: 1.61; 95% CI: 0.79–3.27; p= 0.192), whereas performance status was significantly associated with worse PFS (HR: 3.16; p=0.0091). These results support the findings from the matched analysis and highlight the importance of functional status in this population.
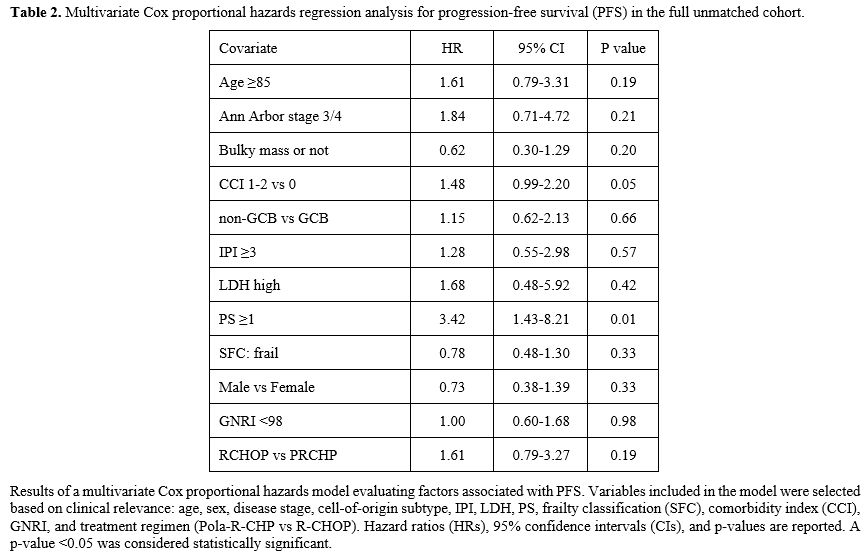 |
|
Safety profiles were comparable between patients with PRCHP and RCHOP, including the rates of grade 3–5 AE (Table 3). Hematological AEs before PSM were neutropenia, anemia, and thrombocytopenia at grades 3–4, observed in 38.1% versus 31.2% (p=0.48), 14.3% versus 27.3% (p=0.07), and 11.1% versus 14.3% (p= 0.62) patients, respectively, in the PRCHP and RCHOP groups. Prophylactic G-CSF was used in the PRCHP (63.5%) and RCHOP (49.4%) groups, and febrile neutropenia was present in the PRCHP (27.0%) and RCHOP (26.0%) groups (p=1.00). Fatigue was the most common non-hematological AE, present in 41.3% of PRCHP and 33.8% of RCHOP (p=0.38), whereas PN was 1.6% of PRCHP versus 13.0% of RCHOP (p=0.01), and was more common in the RCHOP treatment group.
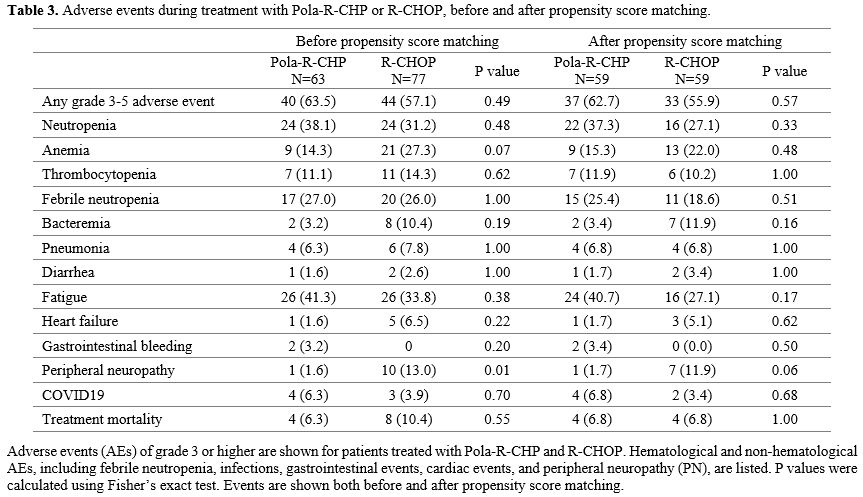 |
|
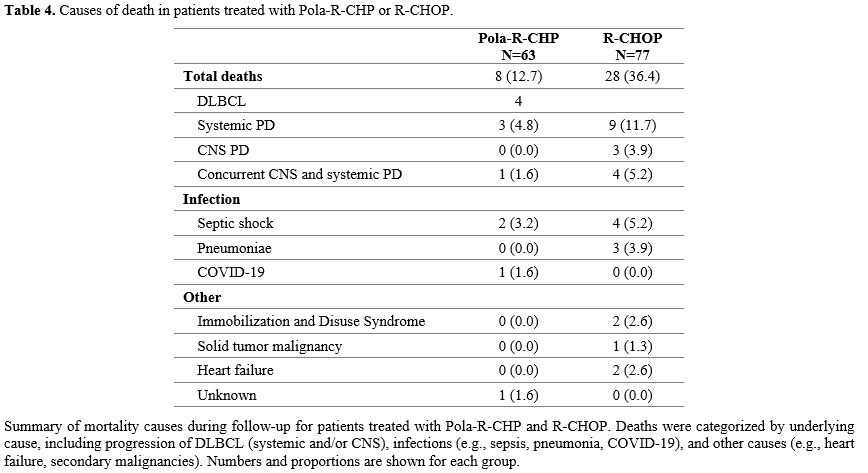
The cause of mortality in the two groups was not comparable due to the different median follow-up durations; however, the progression of DLBCL was the most common, with PRCHP at 6.3% and RCHOP at 20.8% (Table 4).
Discussion
This study is the first to compare dose-attenuated Pola-R-CHP and R-CHOP as first-line therapies in patients over 80 years of age with DLBCL, using PSM based on patient characteristics, including frailty scores, in a single-center retrospective analysis. The study incorporated subgroup analyses according to frailty status and included a real-world evaluation of safety, particularly with respect to PN. The results of the subgroup analysis of the POLARIX trial also showed that for the subgroup aged 75–80 years, PRCHP (n=60) and RCHOP (n=60) were included, with ORR 85.0% and 81.7%, CRR 78.3% and 76.7%, and 2-year PFS 78.9% and 68.9%, respectively, with no significant difference between PRCHP and RCHOP.[15] Zhao et al. conducted a retrospective study comparing PRCHP with RCHOP in a real-world setting and compared patient backgrounds by PSM. They found that the 1-year PFS was 84.1% for RCHOP and 90.3% for PRCHP (HR: 0.538, p= 0.180), indicating that PRCHP performed better, although the difference was not statistically significant.[16] However, no trials have analyzed real-world mini-RCHOP and mini-PRCHP using PSM, especially in older patients aged ≥ 80 years.In clinical practice, a simplified assessment based on careful evaluation of fitness status and comorbidities is crucial for determining the appropriate intensity of treatment. The SFS was developed from a retrospective analysis of a large population-based cohort of older patients with DLBCL, and was shown to be a frailty classification that is easy to use in clinical practice and a predictor of survival and treatment-related mortality in older patients with DLBCL, independent of established prognostic factors and treatment modalities.[12] The SFS can help guide treatment strategies for older patients with DLBCL, and mini-PRCHP is a potential future treatment of choice alongside mini-RCHOP and anthracycline-free regimens. Yagi et al. reported that SFS could accurately predict survival, even in Japanese cohorts, and ARDI had a significant impact on survival outcomes in fit patients with SFC. In contrast, ARDI had no significant impact on survival in non-fit patients.[17] Lee et al. also suggested that in older patients with high frailty, maintaining a high RDI may not lead to prolonged PFS.[18] Isaksen et al. advocated the application of SFS. They found a significant difference in PFS between RCHOP ≥ 80% and ≤ 80% in fit patients (2-year PFS: R-CHOP > 80% vs. R-CHOP ≤ 80%, P=0.002), but no difference was shown between RCHOP ≥ 80% and ≤ 80% in unfit and frail patients.[12] In this study, PRCHP appeared to be associated with improved survival compared to RCHOP in non-frail patients as classified by the SFS. However, given the limited sample size and wide confidence intervals, this finding should be interpreted with caution. No clear survival benefit was observed in frail patients between the two regimens. In patients with high frailty, increasing the RDI or switching to Pola did not influence survival, as the risk of TRM is high. However, according to Isaksen et al., a difference in PFS could be observed in anthracycline-free vs. ARDI ≤ 80% regimens for both the unfit and frail groups (unfit group; 2-year PFS: R-CHOP 80% vs. anthracycline-free, p<0.001, frail group; 2-year PFS: R-CHOP ≤ 80% vs. anthracycline-free, p=0.007), and anthracycline should be administered as much as possible to frail patients if PFS/OS is to be prolonged.[13] Lee et al. conducted a retrospective multicenter analysis of 127 patients with DLBCL aged 80 years or older treated with R-CHOP and found that the 2-year survival rate was significantly higher in the group with >50% ARDI (61.8% vs. 50.8%, p= 0.01).[18] A Netherlands Cancer registry dataset comparing R-CHOP and mini-RCHOP with PSM, with individuals ≥65 years defined as ageing patients, showed a 2-year OS of 60% for R-mini-CHOP vs. 75% for R-CHOP (p<0.012).[19] The 2-year survival rates in this study were 82.8% for PRCHP and 68.8% for RCHOP, which were better, and included half of the frail patients. This may be because, among clinical trials, in real-world practice, every cycle dose-adjustment is free, and patients can start with mini-RCHOP or mini-PRCHP; dose-up if there are no AEs, and can dose-down if AEs occur. In addition, the dose of VCR was reduced in all cases; however, Pola could be administered almost without dose reduction due to its antibody drug properties.
Moreover, a formal interaction analysis was attempted, but could not be estimated due to the absence of Pola-R-CHP cases prior to its approval in August 2022. This supports the interpretation that the observed PFS advantage in non-frail patients receiving Pola-R-CHP is unlikely to be due to the treatment era.
Furthermore, to assess the robustness of our findings, we performed a sensitivity analysis using a multivariate Cox proportional hazards model in the entire pre-matched cohort. After adjusting for clinically relevant covariates, including age, sex, frailty classification, PS, stage, IPI, LDH, GNRI, and comorbidities, the treatment regimen (PRCHP vs RCHOP) was not independently associated with PFS. However, performance status (≥1) remained significantly associated with poorer PFS. These findings suggest that, beyond the type of treatment, functional status may be a critical determinant of prognosis in very elderly patients.
In the POLARIX trial, the safety profile was similar in both groups,[7] as well as in the PN group.[20] Moreover, the Polar-BEAR trial, which is ongoing, includes frail patients aged 75–80 years and those over 80 years, and interim reports show that the frequency of infections and PN in PRCHP and RCHOP is comparable.[9] In this study, hematological AEs and febrile neutropenia were equally frequent with PRCHP and RCHOP; however, PRCHP resulted in fewer AEs than RCHOP, particularly for PN. PN should be avoided whenever possible, especially in older and frail patients, as it increases the risk of falls, is sometimes severe enough to require psychological support, and may lead to reduced ADL and quality of life.[21]
The risk of PN in this study appeared lower with PRCHP than with RCHOP; however, the retrospective design limits the ability to attribute causality to either VCR or Pola. Previous reports suggest that the risk of developing PN due to Pola is related to low albumin and high body weight,[22,23] whereas VCR-induced PN is reported to be related to age, rather than weight.[24] In the POLARIX trial, the median weight was 74.2 kg (range: 38.4–228.0 kg),[22] whereas in this study, median weight of the PRCHP group was 50 kg (range: 32–74 kg), suggesting that older patients with low body weight, such as Asians, may benefit as much or more from Pola than from VCR, especially with RCHOP while reducing the incidence of PN.[25] These differences in demographic characteristics may potentially influence the tolerability profile of these agents, but this conclusion remains speculative. Furthermore, Liao et al. reported that no dose adjustment is required to maintain efficacy and safety in terms of pharmacokinetics between Asians and non-Asians.[26] Given the retrospective nature of AE capture, it is difficult to distinguish whether the observed PN events were definitively attributable to VCR or Pola, and further prospective studies are needed to clarify this. Fatigue is a frequent AE in older individuals with high frailty and can occur even with disease control, sometimes making it difficult to continue and prevent treatment. Dose adjustment of chemotherapy may be necessary.[4]
Importantly, this was a single-center, retrospective study with a small sample size and a relatively short follow-up period. These factors may limit the generalizability of our findings. Furthermore, as a retrospective study, it may be subject to selection bias, potentially overestimating treatment outcomes and limiting applicability to the broader population of older patients with DLBCL, particularly those with frailty. These limitations should be emphasized when interpreting the results. Further prospective multicenter studies with extended follow-up are needed to compare and confirm the efficacy and safety of RCHOP and PRCHP dose reduction therapies in older patients. In addition, the cost-effectiveness of substituting VCR with Pola in older patients warrants further investigation. Japan's universal health insurance system minimizes socioeconomic inequalities in healthcare access. Therefore, although Pola is more expensive than VCR, it is relatively less costly, especially for older patients, and even very old patients can receive it. Japan is the most advanced country worldwide in terms of Pola use. Although the findings of this study may not be directly applicable to other countries, our study, conducted in Japan—the most advanced "super-aged" society worldwide—provides insights that can inform future treatment strategies for DLBCL in other developed nations. Future clinical trials for the treatment of DLBCL using a Pola regimen, including the Polar-BEAR trial, are warranted.
In addition, it is essential to note that the regimens used in this study were not based on predefined protocols, such as R-miniCHOP,[5] but rather reflected physician-adjusted dose adjustments in real-world clinical practice. While we used the term "dose-attenuated PRCHP" and "dose-attenuated RCHOP" for convenience, the actual dosing patterns were individualized and varied according to patient characteristics, tolerability, and physician discretion. This pragmatic approach reflects the flexibility often required when treating very elderly or frail patients outside of clinical trials and may enhance the generalizability of our findings to real-world settings.
Conclusions
Dose-attenuated PRCHP demonstrated efficacy and safety comparable to dose-attenuated RCHOP in older patients and may prolong PFS in non-frail patients, and may be useful for preventing PN.Author contributions
S.S. participated in the statistical analysis, data organization, conceptualization, writing, and revisions of the article. E.S., S.T., and W.K. were responsible for the design, conceptualization, proofreading, and providing suggestions for revisions of the article. S.S. was responsible for the observation and collection of clinical data. T.T. and Y.T. suggested revisions.Data availability
No datasets were generated or analysed during the current study. Declarations, Ethics approval, and consent to participate. This study was approved, and the written informed consent was waived by the Ethics Committee of Shonan Kamakura General Hospital on June 4, 2024 (approval number TGE2422-024), due to its retrospective nature. It was confirmed that the data were anonymized and maintained with confidentiality. The study was conducted in accordance with the Declaration of Helsinki.References
- National Cancer Institute (2015-2019) Cancer stat facts: NHL-diffuse Large B-cell lymphoma (DLBCL). https://seer.cancer.gov/statfacts/html/dlbcl.html Accessed Apr 21, 2023
- Coiffier
B, Lepage E, Briere J, et al (2002) CHOP chemotherapy plus rituximab
compared with CHOP alone in older patients with diffuse large-B-cell
lymphoma. N Engl J Med 346:235-242. https://doi.org/10.1056/NEJMoa011795
PMid:11807147
- Tucci
A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G (2009) A
comprehensive geriatric assessment is more effective than clinical
judgment to identify older diffuse large cell lymphoma patients who
benefit from aggressive therapy. Cancer 115:4547-4553. https://doi.org/10.1002/cncr.24490
PMid:19562776
- Yhim
HY, Park Y, Kim JA, et al (2024) Geriatric risk model for older
patients with diffuse large B-cell lymphoma (GERIAD): A prospective
multicenter cohort study. Korean J Intern Med 39:501-512. https://doi.org/10.3904/kjim.2023.265 PMid:38287501
PMCid:PMC11076889
- Peyrade
F, Jardin F, Thieblemont C, et al (2011) Attenuated immunochemotherapy
regimen (R-miniCHOP) in older patients older than 80 years with diffuse
large B-cell lymphoma: A multicentre, single-arm, phase 2 trial. Lancet
Oncol 12:460-468. https://doi.org/10.1016/S1470-2045(11)70069-9
PMid:21482186
- Bataillard
EJ, Cheah CY, Maurer MJ, Khurana A, Eyre TA, El-Galaly TC (2021) Impact
of R-CHOP dose intensity on survival outcomes in diffuse large B-cell
lymphoma: A systematic review. Blood Adv 5:2426-2437. https://doi.org/10.1182/bloodadvances.2021004665
PMid:33961018 PMCid:PMC8114545
- Tilly
H, Morschhauser F, Sehn LH, et al. (2022) Polatuzumab vedotin in
previously untreated diffuse large B-cell lymphoma. N Engl J Med
386:351-363. https://doi.org/10.1056/NEJMoa2115304
PMid:34904799 PMCid:PMC11702892
- Sato
S, Tsunoda S, Kamata W, Togano T, Tamai Y (2025) Real-world
effectiveness and safety of rituximab and reduced-dose CHP with
polatuzumab vedotin (pola-R-CHP) in patients aged >80 years with
diffuse large B-cell lymphoma: A retrospective analysis. Blood Res
60:10. https://doi.org/10.1007/s44313-025-00059-5
PMid:39907880 PMCid:PMC11799502
- Jerkeman
M, Leppa S, Hamfjord J, Brown P, Ekberg S, Ferreri AJM (2023) Initial
safety data from the phase 3 POLAR BEAR trial in older or frail
patients with diffuse large cell lymphoma, comparing R-Pola-mini-CHP
and R-mini-CHOP. Hemasphere 7:e91359ec https://doi.org/10.1097/01.HS9.0000967820.91359.ec
PMCid:PMC10428412
- N
Nakamura, S Rai, H Sawa, et al (2025) Real-world safety and efficacy of
POLA-R-CHP in patients with previously untreated DLBCL: Analysis of 500
patients in the POLASTAR study. Hematological Oncology 43(Supplement
3). https://doi.org/10.1002/hon.70094_290
- 5th edn. of the
World Health Organization Classification of Haematolymphoid Tumours:
Lymphoid Neoplasms
- Isaksen
KT, Mastroianni MA, Rinde M, et al (2021) A simplified frailty score
predicts survival and can aid treatment-intensity decisions in older
patients with DLBCL. Blood Adv 5:4771-4782. https://doi.org/10.1182/bloodadvances.2021004777
PMid:34543384 PMCid:PMC8759139
- Cheson
BD, Fisher RI, Barrington SF, et al (2014) Recommendations for initial
evaluation, staging, and response assessment of Hodgkin and non-Hodgkin
lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068. https://doi.org/10.1200/JCO.2013.54.8800
PMid:25113753 PMCid:PMC4979083
- Kanda
Y (2013) Investigation of the freely available easy-to-use software
"EZR" for medical statistics. Bone Marrow Transplant 48:452-458. https://doi.org/10.1038/bmt.2012.244
PMid:23208313 PMCid:PMC3590441
- Hu
B, Reagan PM, Sehn LH, et al (2025) Subgroup analysis of older patients
(≥60 years) with diffuse large B-cell lymphoma in the phase 3 POLARIX
study. Blood Adv. https://doi.org/10.1182/bloodadvances.2024014707
PMid:40085955 PMCid:PMC12143816
- Zhao
P, Zhao S, Huang C, et al (2025) Efficacy and safety of polatuzumab
vedotin plus rituximab, cyclophosphamide, doxorubicin and prednisone
for previously untreated diffuse large B-cell lymphoma: A real-world,
multicenter, retrospective cohort study. Hematol Oncol 43: e70017. https://doi.org/10.1002/hon.70017
PMid:39641321
- Yagi
Y, Kanemasa Y, Sasaki Y, et al (2023) Utility of the frailty score for
predicting prognosis and individualizing treatment intensity in older
patients with diffuse large B cell lymphoma. Ann Hematol
102:1485-1500. https://doi.org/10.1007/s00277-023-05233-2
PMid:37115298
- Lee
S, Fujita K, Negoro E, et al (2020) Impact of relative dose intensity
of standard regimens on survival in older patients aged 80 years and
older with diffuse large B-cell lymphoma. Haematologica
105:e415-e418. https://doi.org/10.3324/haematol.2019.234435
PMid:31919079 PMCid:PMC7395278
- Al-Sarayfi
D, Brink M, Chamuleau MED, et al. (2024) R-miniCHOP versus R-CHOP in
older patients with diffuse large B-cell lymphoma: A propensity matched
population-based study. Am J Hematol 99:216-222. https://doi.org/10.1002/ajh.27151
PMid:38014799
- Trněný
M, Fogliato LM, Gardner F, et al (2025) Analysis of peripheral
neuropathy in the POLARIX study using clinician- and patient-reported
outcomes. Blood Adv. https://doi.org/10.1182/bloodadvances.2024014695
PMid:40088467 PMCid:PMC12246594
- Su
YC, Lai YH, Hsieh ST, Teng CJ, Lee YH (2024) Acute, long-term or
non-vincristine-induced peripheral neuropathy among non-Hodgkin
lymphoma survivors: Symptoms, daily activities, functional status, and
quality of life. Eur J Oncol Nurs 69:102540. https://doi.org/10.1016/j.ejon.2024.102540
PMid:38461728
- Lu
D, Gillespie WR, Girish S, et al. (2017) Time-to-event analysis of
polatuzumab vedotin-induced peripheral neuropathy to assist in the
comparison of clinical dosing regimens. CPT Pharmacometr Syst Pharmacol
6:401-408. https://doi.org/10.1002/psp4.12192
PMid:28544534 PMCid:PMC5488137
- Deng
R, Gibiansky L, Lu T, et al (2024) Population pharmacokinetics and
exposure-response analyses of polatuzumab vedotin in patients with
previously untreated DLBCL from the POLARIX study. CPT Pharmacometrics
Syst Pharmacol 13:1055-1066. https://doi.org/10.1002/psp4.13141
PMid:38622879 PMCid:PMC11179702
- Okada
N, Hanafusa T, Sakurada T, et al. (2014) Risk factors for early-onset
peripheral neuropathy caused by vincristine in patients with a first
administration of R-CHOP or R-CHOP-like chemotherapy. J Clin Med Res
6:252-260. https://doi.org/10.14740/jocmr1856w
PMid:24883150 PMCid:PMC4039096
- Triarico
S, Romano A, Attinà G, Capozza MA, Maurizi P, Mastrangelo S, Ruggiero A
(2021) Vincristine-induced peripheral neuropathy (VIPN) in pediatric
tumors: Mechanisms, risk factors, strategies of prevention and
treatment. Int J Mol Sci 22:4112. https://doi.org/10.3390/ijms22084112
PMid:33923421 PMCid:PMC8073828
- Liao
MZ, Deng R, Gibiansky L, et al (2023) Ethnic sensitivity assessment:
Polatuzumab vedotin pharmacokinetics in Asian and non-Asian patients
with previously untreated diffuse large B-cell lymphoma in POLARIX.
Clin Transl Sci 16:2744-2755. https://doi.org/10.1111/cts.13669
PMid:37864313 PMCid:PMC10719464