Inroduction
B-cell prolymphocytic leukemia (B-PLL) is a very rare lymphoproliferative disorder characterized by splenomegaly, lymphocytosis, and cytopenia. It is considered an aggressive disease with a median overall survival of three years.[1,2] The most recent 2022 WHO classification places this condition in a new entity defined as “splenic B-cell lymphoma/leukemia with prominent nucleoli (SBLPN)”, which also includes Hairy Cell Leukemia variant (HCLv) and some cases of other splenic lymphomas. Nevertheless, it remains a distinct entity in the new International Consensus Classification (ICC).[3,4]Morphologically, the presence of atypical lymphocytes, with medium-large basophilic cytoplasm and a round nucleus with a large and prominent nucleolus, is the key diagnostic hallmark. They express common B-cell surface antigens and are negative for Hairy Cell Leukemia (HCL) markers CD25 and CD123.[5] TP53 deletions/mutations were identified in about half of B-PLL/SBPL cases.[6,7]
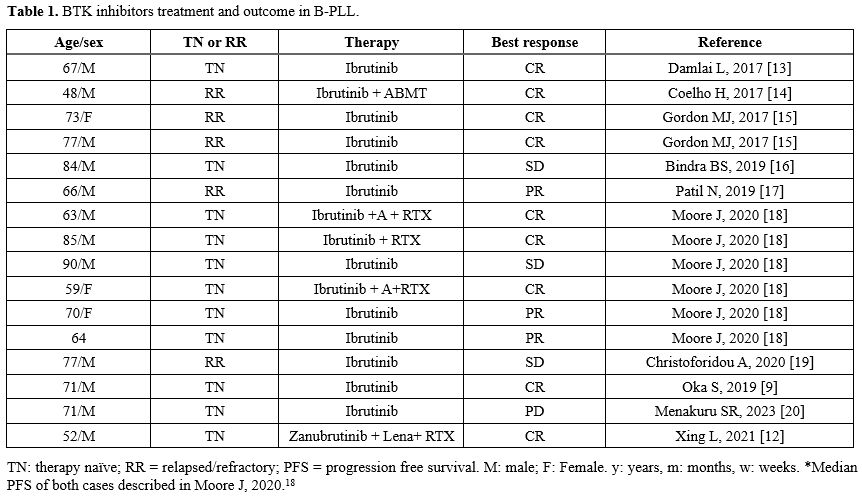
Given the rarity of this disorder, data from clinical trials are completely lacking, and B-PLL/SBLPN is considered an orphan drug disease. As a consequence, treatments for B-PLL/SBLPN substantially derive from the experience of other B-cell lymphoproliferative disorders, in particular from B-CLL. Chemo-immunotherapy responses are generally mild and of short duration. Targeted agents (TAs) now constitute the gold standard for B-CLL treatment. TAs treatment has also shown promising results in B-PLL/SBLPN based on a few experiences describing the use of the first-generation Bruton tyrosine kinase inhibitor (BTKi) Ibrutinib (Table 1),[8-10] obtaining at least partial responses in the setting of chemotherapy-resistant disease. Second-generation covalent BTKi, i.e., Acalabrutinib and Zanubrutinib, have demonstrated in CLL patients a similar efficacy with a better toxicity profile, characterized by a lower frequency of atrial fibrillation and cardiovascular adverse events, and, of importance, good efficacy also in TP53 mutated cases.
Materials and Methods
B-PLL/SBLPN diagnosis was performed according to WHO 2022 and ICC definitions, based on the presence of typical cells with medium-large atypical lymphocytes with basophilic cytoplasm and a round nucleus with a large, prominent nucleolus. Other types of B-cell lymphomas/leukemias were excluded based on FISH, immunophenotyping, and molecular assessments. Blastoid subtype of mantle cell lymphoma was excluded by the absence of t(11;14) and CCND translocations. Moreover, cellular markers CD25 and CD123 were absent, excluding the diagnosis of HCL.Cardiologic assessment and Cumulative Illness Rating Scale-Geriatric (CIRS-G) were used to determine fitness for treatment. Zanubrutinib was administered at 160 mg BID via the BeiGene, named patient program. Hematologic response, spleen size, and treatment tolerance were assessed. Adverse events were graded using NCI CTCAE.
Results
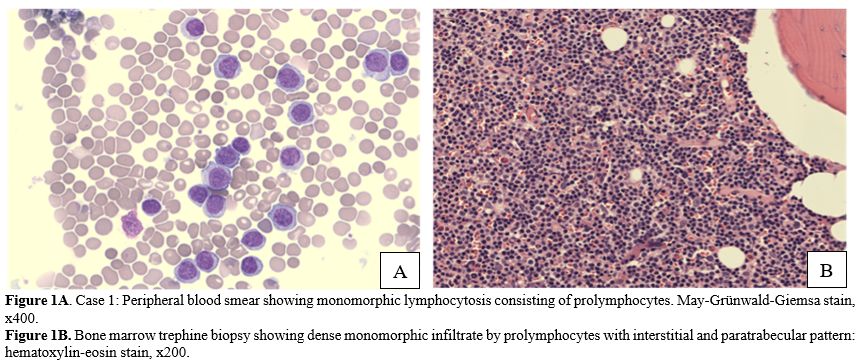
Here, we present two elderly B-PLL/SBLPN patients treated with Zanubrutinib monotherapy, achieving optimal disease control and excellent tolerance.Case 1: An 87-year-old man was first evaluated due to the presence of absolute lymphocytosis with atypical lymphocytes occasionally observed in a blood count analysis. Flow cytometry revealed a clonal B cell population expressing CD19+, CD5+/-, CD23-, CD20+hi, CD22+, FMC7+, CD79b+hi, CD10-, and sIg/k well expressed, equal to 82% of peripheral lymphocytes. The absolute lymphocyte count (ALC) was 29.46x109/L. After a few months, the patient showed a clear increase in ALC, with a white blood count (WBC) of 125.45x10^9/L, ALC of 117.51x10^9/L, hemoglobin (Hb) of 12.9 g/dL, and platelet count (PLT) of 137x10^9/L. The clinical history included post-infarction ischemic cardiopathy, the presence of renal stones, and benign prostatic hypertrophy. CIRS-G score was 10.[11] Testing for the hepatitis B virus demonstrated the presence of anti-core antibodies with HBV-DNA undetectable. Microscopic evaluation of peripheral blood smear revealed the presence of small-to-medium-sized cells with typical basophilic cytoplasm and prominent nucleoli in the great majority of the circulating lymphocytes (Figure 1A). At physical examination, no hepatosplenomegaly was revealed, small (< 1 cm) bilateral inguinal lymph nodes were present, and no other significant anomalies were noted. A baseline abdominal ultrasound showed mild splenomegaly (bipolar diameter of 14.4 cm).
Afterwards, in the next 3 months, we observed a further rapid increase in WBC and the onset of mild anemia (Hb 12.5 g/dl). Fluorescence in situ hybridization (FISH) analysis revealed the presence of del(17p) and del(13q14), and the absence of del(11q22), tris(12), and t(11;14). Karyotyping revealed a highly complex karyotype (42~46, XY, -2, -5, -6, -7, ?i(7)(q10), +der(8)add(8)(q24), add(9)(p24), -10, add(12)(p13), -13, der(18)t(13;18)(q?14;q?23), add(19)(?q13), -20, -21, -22, +2~5mar[cp20]) defined as “composite” due to the presence of subclones with different alterations. Molecular analysis through Sanger sequencing highlighted the presence of Val173Ala TP53 missense mutation in exon 7. The immunoglobulin heavy-chain variable region gene (IGHV) was defined as mutated.
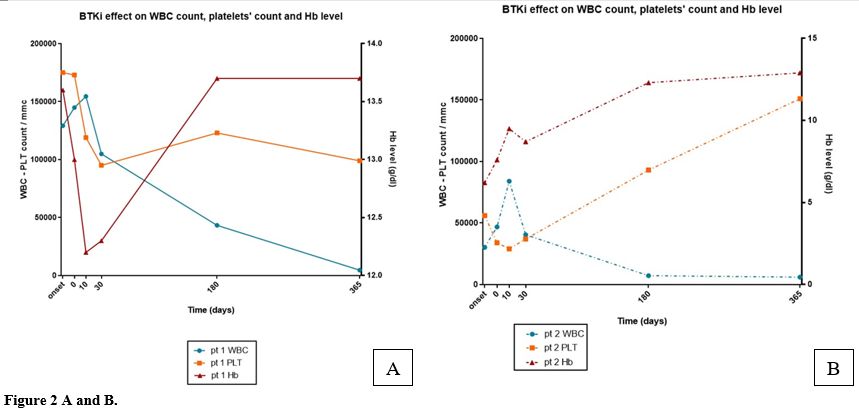
Cardiologic assessment showed a stable cardiologic disease in chronic ischemic cardiopathy with hypertension; no significant abnormalities at the echocardiography were revealed, and the ejection fraction (EF) of the left ventricle was normal (EF 62%). In April 2023, he started treatment with the 2nd-generation BTK covalent inhibitor Zanubrutinib at standard dosage (160 mg BID). We observed an initial very mild increase of ALC (146.30x109/L vs 135.40x109/L) after 2 weeks of treatment with a subsequent progressive low decrease. At the end of the first month of treatment, ALC was 98.29x10^9/L. A normal WBC was obtained after 1 year of treatment (Figure 2A). At the 6th month of therapy, an abdominal ultrasound examination showed a reduction of the spleen dimension (bipolar diameter equal to 11.3 cm). Mild thrombocytopenia occurred (PLT99x109/L), corresponding to a Grade 1 adverse event according to NCI CTCAE, not associated with any clinical manifestation and not requiring dose adjustment or temporary treatment discontinuation. After two years, the response was defined as a good partial response, showing complete normalization of the WBC, ALC, and Hb level, and a PLT count of 110x109/L/L. Flow cytometry showed a low persistence of prolymphocytes (7% of ALC equal to 2.87x109/L).
Case 2: An 80-year-old woman presented with an ALC of 20.12x109/L, severe anemia with an Hb level of 7.6 g/dl, and thrombocytopenia (PLT of 34x109/L). She had a history of hypertension treated with an ACE inhibitor (ramipril 5mg/day) and dyslipidemia treated with simvastatin 10 mg/day. CIRS-G score was 6. Flow cytometry showed a clonal B cell population corresponding to 90% of the total ALC of 20.840x109/L expressing CD5, CD19, CD20, CD22, CD79b, sIg/k and negative for CD23. On physical examination, splenomegaly was assessed at 8 cm from the costal arch; no superficial lymph nodes were palpable. FISH analysis revealed the presence of both del(17p) and del(11q22); translocation t(11;14) was absent, excluding the diagnosis of mantle cell lymphoma. Molecular analysis through Sanger sequencing showed the presence of Cys238PheTP53 mutation in exon 7. Karyotyping showed a highly complex karyotype (4~46, X, -X, del(2)(p?21), -2, del(6)(q22), der(9)t(2;9)(q?21;p?21), +der(11)t(11;17)(q?13;q?21), +1~2mar[cp20]) defined by the presence of subclones with different alterations, thus named “composite”. Peripheral blood smear and bone marrow biopsy showed infiltration of atypical medium-sized lymphocytes with basophilic cytoplasm and a prominent central or eccentric nucleolus in inter- and para-trabecular nodular aggregates without fibrosis (Figure 1B). A specialistic cardiologic assessment was performed, and echocardiography showed moderate left atrial enlargement and mild mitral insufficiency with normal EF (62%).
Zanubrutinib was started at standard dosage (160 mg BID). At this time, the peripheral blood count revealed WBC of 30.46x10^9/L, neutrophil count (ANC) of 1.16x10^9/L, and ALC of 28.82x10^9/L/L. Hb was g/dL,g/dl, andwas 56x10^9/L109/L/L. Initially, a low increase in the lymphocyte count and a further reduction in the neutrophil count with an ANC of 0.55 x109/L were observed. Afterwards, we observed a slow but progressive ALC reduction, obtaining a substantial complete normalization of the leukocyte count. After 3 months, Hb was 12.6 g/dl, with normalization of ALC and ANC. Platelet level improved more slowly, achieving a PLT of 119x109/L at the 6th month of treatment (Figure 2B). The splenic pole was not palpable after 2 months of treatment. After 15 months, at the time of the last observation, she remained in good partial remission with marked reduction of the prolymphocytic B population, equal to8% prolymphocytes of total lymphocytes, normalization of the WBC equal to 4.54x109/L, ANC of 2.45x109/L, ALC of 1.75x109/L, Hb of 13.3 gr/dl, and PLT of 142x109/L.
During the treatment, blood pressure levels worsened, requiring anti-hypertensive therapy adjustment (G2), adding amlodipine 5 mg/day associated with ramipril without any clinically relevant episode. The patient reported two episodes of palpitation, no alteration of cardiac frequency and rhythm was revealed in the different 12-lead and Holter ECG.
Discussion
Here, we report our experience concerning two very elderly cases diagnosed with B-PLL/SBPLN based on morphological and immunophenotypic characteristics. A highly complex karyotype, including del(17p) and TP53 alterations, was revealed in accordance with the few B-PLL cases reported.[6] Based on a good cardiovascular safety profile of the drug, we deemed the use of Zanubrutinib as a single agent adequate, obtained through a Beigene named patient program.Zanubrutinib is a second-generation covalent BTKi, approved in the US and Europe for CLL, mantle cell lymphoma, and Waldenström disease treatment. No significant reports describing the activity and tolerability of this compound as continuous single-agent treatment in B-PLL/SBLPN are available.[12] Continuous therapy with BTKi seems a suitable treatment for PLL on the basis of previous, although limited, experiences reported, in which a good control of the disease was reached. Because second-generation BTKi revealed better tolerability compared to ibrutinib, we offered treatment with zanubrutinib for these very elderly B-PLL patients, avoiding the combination with other potentially active drugs that could potentially increase the risk of adverse events.
After a mild increase in the peripheral ALC, both patients obtained a good partial response, as expected with BTKi continuous treatment. Notably, case 2 also showed a progressive improvement of all hematological parameters, with the increase and normalization of the hemoglobin level and platelet count. A very good response was also obtained for the spleen size in both patients. Toxicity was very mild; no temporary suspension of the treatment or dose reduction was necessary for either patient, after a follow-up of 24 months for case 1 and 15 months for case 2. No significant alterations of heart frequency and rhythm were observed; only a mild worsening of blood pressure level occurred in case 2, which was easily controlled.
Conclusions
These two cases suggest the utility and safety of upfront therapy with Zanubrutinib even in elderly/very elderly patients, suggesting that this compound should be considered for upfront therapy of B-PLL/SBLPN.Author Contributions
SG and MR designed the work, collected data, and wrote the original draft. All Authors were involved in the clinical management of the patients, revised and approved the final version of the manuscript.Acknowledgments
We especially thank the patients who gave their consent to publish this report. The authors thank dr Giulia Debbia for data management and her skilful assistance with the ethical committee submission. The authors acknowledge Beigene for providing Zanubrutinib free of charge through a named-patient programme.Data Availability Statement
No data associated with our study has been deposited in a publicly available repository. All data generated or analyzed are included in this published article.Ethics approval statement
This case series was approved by the Ethics Committee of Area Vasta Emilia Nord (Comitato Etico Varia Vasta Emilia Nord), with protocol number AOU 0006664/25, dated 05 March 2025. Written informed consent was obtained from the patients included.References
- Catovsky D, Galetto J, Okos A, et al. Prolymphocytic leukaemia of B and T cell type. Lancet 1973; 2: 232-234. https://doi.org/10.1016/S0140-6736(73)93135-8 PMid:4124423
- Shvidel
L, Shtalrid M, Bassous L, et al. B-Cell Prolymphocytic Leukemia: A
Survey of 35 Patients Emphasizing Heterogeneity, Prognostic Factors and
Evidence for a Group with an Indolent Course. Leukemia & Lymphoma
1999; 33: 169-179.
https://doi.org/10.3109/10428199909093739 PMid:10194135 - Alaggio
R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World
Health Organization Classification of Haematolymphoid Tumours: Lymphoid
Neoplasms. Leukemia 2022; 36: 1720-1748. https://doi.org/10.1038/s41375-022-01620-2 PMid:35732829 PMCid:PMC9214472
- Campo
E, Jaffe ES, Cook JR, et al. The International Consensus Classification
of Mature Lymphoid Neoplasms: a report from the Clinical Advisory
Committee. Blood 2022; 140: 1229-1253. https://doi.org/10.1182/blood.2022019016 PMCid:PMC10023734
- El
Hussein S, Khoury JD, Medeiros LJ. B-prolymphocytic leukemia: Is it
time to retire this entity? Annals of Diagnostic Pathology 2021; 54:
151790. https://doi.org/10.1016/j.anndiagpath.2021.151790 PMid:34293709
- Chapiro
E, Pramil E, Diop M, et al. Genetic characterization of B-cell
prolymphocytic leukemia: a prognostic model involving MYC and TP53.
Blood 2019; blood.2019001187. https://doi.org/10.1182/blood.2019001187 PMid:31527074
- Lens
D, J.J.C. De Schouwer P, Hamoudi RA, et al. p53 Abnormalities in B-Cell
Prolymphocytic Leukemia. Blood 1997; 89: 2015-2023. https://doi.org/10.1182/blood.V89.6.2015 PMid:9058723
- Mouhssine
S, Maher N, Matti BF, et al. Targeting BTK in B Cell Malignancies: From
Mode of Action to Resistance Mechanisms. IJMS 2024; 25: 3234. https://doi.org/10.3390/ijms25063234 PMid:38542207 PMCid:PMC10970225
- Oka
S, Ono K, Nohgawa M. Effective upfront treatment with low-dose
ibrutinib for a patient with B cell prolymphocytic leukemia. Invest New
Drugs 2020; 38: 1598-1600. https://doi.org/10.1007/s10637-020-00902-9 PMid:31965420
- Jain A, Khunger J, Prasad P, et al. An illustrative case of B-cell prolymphocytic leukemia. Blood Res 2020; 55: 181-184. https://doi.org/10.5045/br.2020.2020079 PMid:32883890 PMCid:PMC7536564
- Salvi
F, Miller MD, Grilli A, et al. A Manual of Guidelines to Score the
Modified Cumulative Illness Rating Scale and Its Validation in Acute
Hospitalized Elderly Patients. J American Geriatrics Society 2008; 56:
1926-1931. https://doi.org/10.1111/j.1532-5415.2008.01935.x PMid:18811613
- Xing
L, He Q, Xie L, et al. Zanubrutinib, rituximab and lenalidomide induces
deep and durable remission in TP53-mutated B-cell prolymphocytic
leukemia: a case report and literature review. haematol 2021; 107:
1226-1228. https://doi.org/10.3324/haematol.2021.280259 PMid:34937321 PMCid:PMC9052906
- Damlaj
M, Al Balwi M, Al Mugairi AM. Ibrutinib therapy is effective in B-cell
prolymphocytic leukemia exhibiting MYC aberrations. Leuk Lymphoma 2018;
59: 739-742. https://doi.org/10.1080/10428194.2017.1347653 PMid:28695755
- Coelho
H, Badior M, Melo T. Sequential Kinase Inhibition
(Idelalisib/Ibrutinib) Induces Clinical Remission in B-Cell
Prolymphocytic Leukemia Harboring a 17p Deletion. Case Rep Hematol
2017; 2017: 8563218. https://doi.org/10.1155/2017/8563218 PMid:28819574 PMCid:PMC5551464
- Gordon
MJ, Raess PW, Young K, et al. Ibrutinib is an effective treatment for
B-cell prolymphocytic leukaemia. Br J Haematol 2017; 179: 501-503. https://doi.org/10.1111/bjh.14224 PMid:27391978
- Bindra
BS, Kaur H, Portillo S, et al. B-cell Prolymphocytic Leukemia: Case
Report and Challenges on a Diagnostic and Therapeutic Forefront. Cureus
2019; 11: e5629. https://doi.org/10.7759/cureus.5629
- Patil
N, Went RG. Venetoclax is an option in B-cell prolymphocytic leukaemia
following progression on B-cell receptor pathway inhibitors. Br J
Haematol 2019; 186: e80-e82. https://doi.org/10.1111/bjh.15912 PMid:30941750
- Moore
J, Baran AM, Meacham PJ, et al. Initial treatment of B-cell
prolymphocytic leukemia with ibrutinib. Am J Hematol 2020; 95:
E108-E110. https://doi.org/10.1002/ajh.25733
- Christoforidou
A, Bezirgiannidou Z, Vrachiolias G, et al. B-cell prolymphocytic
leukemia successfully treated with B-cell receptor antagonists, but
resistant to venetoclax. Leuk Lymphoma 2020; 61: 749-752. https://doi.org/10.1080/10428194.2019.1689392 PMid:31713446
- Menakuru SR, Roepke J, Siddiqui S. De-Novo B-Cell Prolymphocytic Leukemia. J Hematol 2023; 12: 82-86. https://doi.org/10.14740/jh1096 PMid:37187496 PMCid:PMC10181324