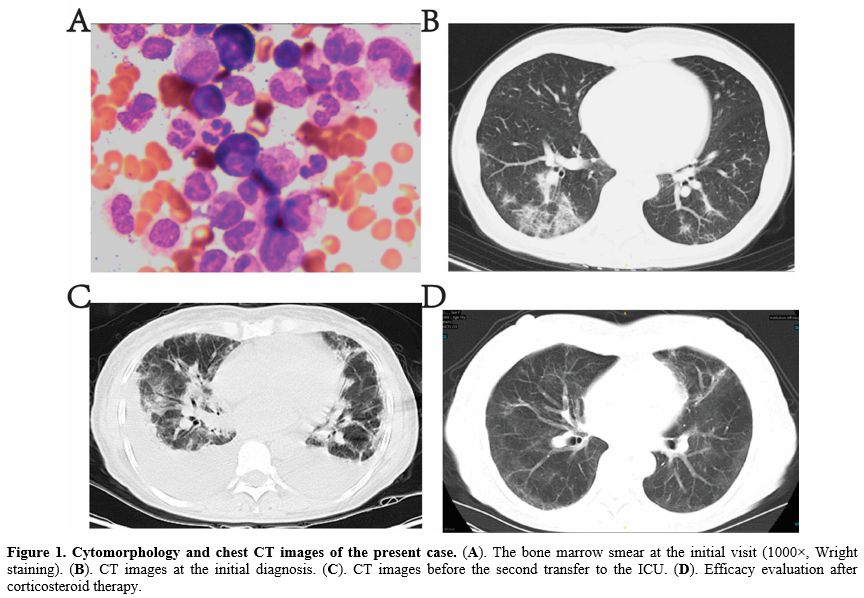
A 56-year-old woman presented with fever (38.5°C), cough, yellow sputum, and fatigue, without palpitations, chest tightness, or dyspnea. Blood tests at another institution revealed a white blood cell count of 279.68×10⁹/L, hemoglobin of 85 g/L, and platelets of 619×10⁹/L. Oral moxifloxacin was administered but showed no improvement. Bone marrow aspiration at our hospital indicated hypercellular marrow with marked granulocytic hyperplasia (predominantly mid-to-late-stage granulocytes), eosinophils, and basophils. Erythroid lineage hyperplasia was poor. Megakaryocytes exceeded 100 per slide (Figure 1A) Karyotype analysis revealed 46, XX,t(9;22)(q34;q11). PCR confirmed BCR-ABL1 p210 fusion gene positivity. Arterial blood gas analysis showed PaO₂ 43.0 mmHg (normal: 83–108 mmHg), PaCO₂ 34.0 mmHg (normal: 83–108 mmHg), and pH 7.47 (normal: 7.35–7.45). Chest CT suggested interstitial pneumonia (Figure 1B). Testing positive for H1N1 influenza A virus antigen via nasal swab and demonstrating positive serum IgM antibodies against H1N1, the patient was diagnosed with CML complicated by H1N1 virus infection, pulmonary infection, and respiratory failure. Treatment included hydroxyurea, imatinib, oseltamivir, and meropenem.
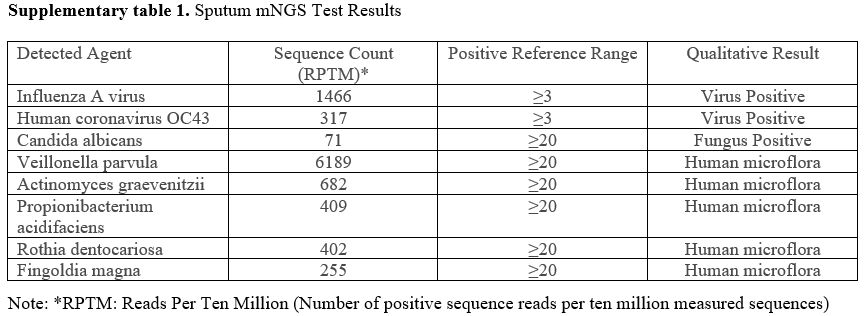
Subsequently, the patient’s oxygen saturation dropped to 83-95%, requiring transfer to the ICU. Sputum metagenomic Next-Generation Sequencing (mNGS) detected H1N1 influenza A virus and human coronavirus OC43 (Supplementary table 1). High-flow nasal oxygen therapy, leukocyte reduction, and intensified antimicrobial therapy (oseltamivir, meropenem, moxifloxacin, posaconazole) were initiated. After stabilization, she returned to the hematology department, but chest CT showed no improvement. Subsequent sputum cultures revealed carbapenem-resistant Acinetobacter baumannii (CRAB) and Stenotrophomonas maltophilia. Multiple antibiotics (moxifloxacin, biapenem, posaconazole, sulbactam, meropenem, caspofungin, vancomycin, tigecycline) were administered, yet recurrent fever and dyspnea persisted. Ten days later, chest CT showed progression of bilateral interstitial pneumonia and pleural effusion (Figure 1C). She was readmitted to the ICU.
In the ICU, high-flow oxygen therapy and methylprednisolone (60 mg/day) were administered without further antibiotics. Imatinib was continued for CML. Her body temperature normalized, and dyspnea resolved within days. Chest CT 16 days later demonstrated significant improvement in pulmonary lesions (Figure 1D).
Data on H1N1 infection in chronic-phase CML patients are scarce. Adel et al. investigated the 64-slice multidetector computed tomography (MDCT) findings in 12 patients with hematological malignancies co-infected with the H1N1 virus. The study found that all patients exhibited multiple pulmonary lesions. Among these, airway wall thickening/dilatation (in all 12 patients), ground-glass opacities (GGO, 9/12), nodules (6/12), and consolidation (6/12) were the most common findings. The lesions were mostly bilateral, with more significant involvement of the left lung and lower lobes, and were predominantly distributed along the peribronchovascular regions. Acute myeloid leukemia (AML) was the most common type of underlying hematological malignancy (8 cases), with the remaining cases being chronic lymphocytic leukemia, multiple myeloma, and myelodysplastic syndrome. No cases of CML were observed.[1]
Two cases of post-transplant H1N1 infection in CML patients have been reported: a 35-year-old male with a history of Allo-HSCT (3 years prior) and discontinued immunosuppression recovered uneventfully,[2] while a 54-year-old male in blast crisis with molecular relapse and lung aspergillosis died.[3] However, no studies on community-acquired H1N1 infection in chronic-phase CML patients exist.
Our case describes a chronic-phase CML patient whose initial presentation was severe H1N1-related interstitial pneumonia requiring two ICU admissions. Despite sputum cultures indicating bacterial co-infection, antibiotics alone failed to halt disease progression. Glucocorticoid therapy led to marked improvement, suggesting an immune-mediated post-viral infection.
In this patient, pneumonia occurred before the diagnosis of CML. Following the diagnosis, the patient received treatment with imatinib. Some studies suggest that imatinib may impair the functions of various cells involved in the immune response, particularly cell-mediated immunity.[4,5] Breccia et al. evaluated the infection risk in patients with chronic-phase CML treated with imatinib. The results showed an overall infection rate of 14%, primarily consisting of herpes zoster (8.4%) and pneumonia (2.8%). Their study indicates that while imatinib may affect cellular immunity by reducing lymphocyte counts, it does not significantly increase the risk of severe infections in chronic-phase CML patients. Herpes zoster reactivation was the most common, yet manageable, complication, and routine prophylaxis is not required.[6]
Tyrosine kinase inhibitor (TKI) therapy for CML may also cause non-infectious pulmonary complications, including interstitial lung disease. While the incidence of TKI-related ILD is unclear, cases of imatinib-associated interstitial pneumonia have been reported. Histologic analysis may reveal cytotoxic or non-cytotoxic lung injury.[7,8] In this case, pulmonary lesions predated TKI initiation but later improved with antimicrobial and corticosteroid therapy during continued imatinib treatment. This outcome suggests that TKI did not appear to contribute to the lung injury.
In conclusion, we present a rare case of chronic-phase CML with H1N1-related interstitial pneumonia as the initial presentation. Further large-scale epidemiological studies are needed to clarify the characteristics of H1N1 infection in this population.
Acknowledgments
We express our sincere appreciation to Dr. XL Hu for her invaluable guidance.Consent to participate
Informed consent was obtained from the patient.Author contributions
Zhan Su and Feng Wang conceived and designed the study and were responsible for drafting the manuscript. All authors critically reviewed and approved the final version of the manuscript for publication.References
- El-Badrawy A, Zeidan A, Ebrahim MA (2012) 64
multidetector CT findings of influenza A (H1N1) virus in patients with
hematologic malignancies. Acta Radiol 53:662-667. https://doi.org/10.1258/ar.2012.120038 PMid:22734081
- Lalayanni
C, Sirigou A, Iskas M, et al (2010) Outbreak of novel influenza A
(H1N1) in an adult haematology department and haematopoietic cell
transplantation unit: Clinical presentation and outcome. Journal of
Infection 61:270-272. https://doi.org/10.1016/j.jinf.2010.06.013 PMid:20600296
- Garland
P, De Lavallade H, Sekine T, et al (2011) Humoral and Cellular Immunity
to Primary H1N1 Infection in Patients with Hematologic Malignancies
following Stem Cell Transplantation. Biology of Blood and Marrow
Transplantation 17:632-639. https://doi.org/10.1016/j.bbmt.2010.08.002 PMid:20708085
- Sinai
P, Berg RE, Haynie JM, et al (2007) Imatinib Mesylate Inhibits
Antigen-Specific Memory CD8 T Cell Responses In Vivo. The Journal of
Immunology 178:2028-2037. https://doi.org/10.4049/jimmunol.178.4.2028 PMid:17277106
- Chen
CI-U, Maecker HT, Lee PP (2008) Development and dynamics of robust
T-cell responses to CML under imatinib treatment. Blood 111:5342-5349. https://doi.org/10.1182/blood-2007-12-128397 PMid:18326818 PMCid:PMC2396727
- Breccia
M, Girmenia C, Latagliata R, et al (2011) Low incidence rate of
opportunistic and viral infections during imatinib treatment in chronic
myeloid leukemia patients in early and late chronic phase. Mediterr J
Hematol I 3:e2011021. https://doi.org/10.4084/mjhid.2011.021 PMid:21713076 PMCid:PMC3113277
- Choi
MH, Jung JI, Chung WD, et al (2014) Acute Pulmonary Complications in
Patients with Hematologic Malignancies. RadioGraphics 34:1755-1768. https://doi.org/10.1148/rg.346130107 PMid:25310429
- Kim S-A, Kwon BS, Chung J-H, et al (2022) Interstitial pneumonitis associated with dasatinib treatment for chronic myeloid leukemia or acute lymphoblastic leukemia: case series and a literature review. Ther Adv Respir Dis 16:17534666221135322. https://doi.org/10.1177/17534666221135322 PMid:36346055 PMCid:PMC9647296
Supplementary Files