Conventional coagulation assays (CCAs) and platelet count (Plts) provide minimal information on the hemostatic process and are not good predictors of bleeding and thrombotic risk. Global coagulation assays (GCAs), which allow a comprehensive analysis of the entire hemostatic process, may provide more information. However, these tests are not routinely performed.
Recently, Raso et al.[7] have shown the complexity of hemostatic alterations in acute myeloid leukemia (AML) through thromboelastography (TEG), a GCA that measures the viscoelastic properties of the clot, providing information from the clot formation to fibrinolysis.[8] However, further evidence is required to determine its potential usefulness in this context.
Another promising test is represented by the thrombin generation assay (TGA), which evaluates the formation and decay of thrombin upon activation of the coagulation cascade by tissue factor (TF).[9] Recently, Betticher et al.[10] published their experience about the application of TGA in a pediatric cohort diagnosed with acute lymphoblastic leukemia (ALL), reporting that this assay, evaluated during induction treatment, is a predictor of thrombotic complications. However, additional research is necessary to clarify its role in this setting.
In our center, a prospective observational monocentric pilot study was performed to evaluate the possible association between TGA parameters at diagnosis and thrombotic risk during follow-up in patients with acute leukemia (AL).
Materials and Methods
Study population. Eligible patients were subjects > 18 years of age diagnosed with AML, acute promyelocytic leukemia (APL), mixed phenotype acute leukemia (MPAL), and ALL as per international guidelines[11-14] diagnosed between February 2022 and September 2024 in the Department of Translational and Precision Medicine of Sapienza University of Rome. Anticoagulant and antiplatelet therapy at the time of AL diagnosis constituted an exclusion criterion. All enrolled subjects provided informed consent according to the principles of the Declaration of Helsinki.Clinical characteristics and data collection. Baseline characteristics, including age, sex, AL subtype, previous history of VTE, and laboratory parameters, were systematically recorded in a secure, study-specific database accessible exclusively to authorized study personnel. Patients were prospectively followed for the occurrence of thrombotic events until the last follow-up or death from any cause. Thrombotic events were defined as VTE, superficial venous thrombosis (SVT), or arterial thrombosis. Suspected venous thrombotic events were confirmed by appropriate imaging modalities, including Doppler ultrasound and/or contrast-enhanced computed tomography with iodinated contrast media. Arterial thrombotic events were confirmed using computed tomography angiography.
Laboratory evaluation. At the time of AL diagnosis, a baseline assessment of complete blood counts (CBCs), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (Fg), anti-thrombin (AT), and TGA was performed. Blood samples were collected using either a 19–21-gauge needle or a central venous catheter (CVC), with the first 5 mL discarded; then they were analyzed within 2 hours after collection. Coagulation samples were collected in citrated blood [anticoagulated with 109 mmol/l (3.2%) trisodium citrate] in the absence of any concomitant anticoagulant treatment.
Thrombin generation assay. The thrombin generation assay, a global coagulation test that reproduces the kinetics of thrombin formation, was performed using the Calibrated Automated Thrombogram (CAT) (Diagnostica Stago, Asnières, France) according to the manufacturer’s specifications. Twenty-two plasma samples per plate were mixed with assay reagents (tissue factor and phospholipids), and the fluorescent signal indicating TG was monitored in a Fluoroskan Ascent Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Parameters were calculated with the Thrombinoscope Software Program (Thrombinoscope BV, Maastricht, The Netherlands). The following parameters were included: thrombin peak, which represents the maximum concentration of thrombin formed at any time; endogenous thrombin potential (ETP), which depicts the total amount of thrombin generated over time and reflects the total enzymatic activity of thrombin; time to peak (ttPeak), which indicates the time required to reach the thrombin peak; the lag time, which measures the time between the start of the assay and the initial formation of TG; the velocity index, which is defined as (peak height/[time to peak-lag time]) indicating the rate of TG formation.
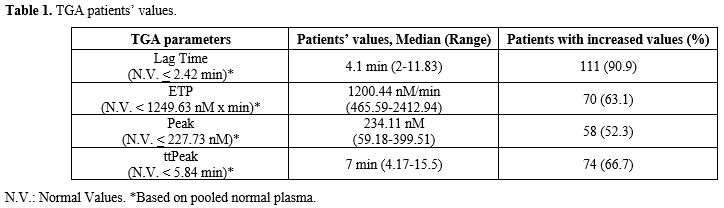
TGA parameters' normal values were established based on the analysis of pooled normal plasma (Table 1).
Complete blood count and conventional coagulation assays. Complete blood count was performed using an ADVIA 2120 analyzer (Siemens, Munich, Germany); PT, APTT, and AT were performed using the automated coagulometer BCS Xp (Siemens), and Fg was assayed by the Clauss method using reagents and methodology according to the manufacturer’s instructions.
All blood samples were collected and processed within 2 hours. The tubes were centrifuged for 10 minutes at 4500 rpm.
Statistics. We performed a descriptive analysis of our case series, including continuous variables expressed in terms of median and range, and categorical variables expressed as frequencies. Overall survival (OS) has been defined as the time from the AL diagnosis to the last follow-up or death for any cause. Event-free survival (EFS) was calculated as the time from the AL diagnosis to the onset of adverse thrombotic events (T-AEs), when they occurred. The cumulative incidence of thrombosis was depicted with the 1-KM method. Chi-square analysis and Mann-Whitney U test were performed to evaluate the differences between subgroups. Cox regression was performed to evaluate the association between the development of thrombotic events, TGA parameters, and other laboratory values, when appropriate. Statistical significance was considered for values of p < 0.05. Statistical analysis was performed with the “IBM SPSS Statistics” software version 26.
Results
Patients’ baseline characteristics and AL treatment. Patients’ baseline characteristics are summarized in Table 2. One hundred and eleven patients were enrolled: 58 (52.3%) were females and 53 (47.7%) were males. Two patients (1.8%) experienced an episode of VTE before the diagnosis of AL. Median age at AL diagnosis was 63.6 years (range 18-86.4). Eighty-three patients (74.8%) were diagnosed with AML (APL-excluded), 15 (13.5%) with ALL, 8 (7.2%) with APL, and 5 (4.5%) with MPAL. At diagnosis, CVC was placed in 63 patients (56.8%); median bone marrow blast count was 60% (range 10-100%); median CBC values were hemoglobin 9.5 g/dL (range 4.5-14.6), white blood cells 6.03*103/mmc (range 0.44-116.7), platelets 66*103/mmc (range 3-361). Median values of CCAs were PT ratio 1.06 (range 0.8-3.32), APTT ratio 0.96 (range 0.73-1.6), Fg 363 mg/dL (range 54-1026), AT 94.5% (range 55-126). Thrombin generation assay median parameters were Lag Time 4.1 min (range 2-11.83), ETP 1200.44 nM x min (range 465.59-2412.94), Peak 234.11 nM (range 59.18-399.51), ttPeak 7 min (range 4.17-15.5) (Figure 1-2). Median follow-up was 8.28 months (range 0.03-32.4).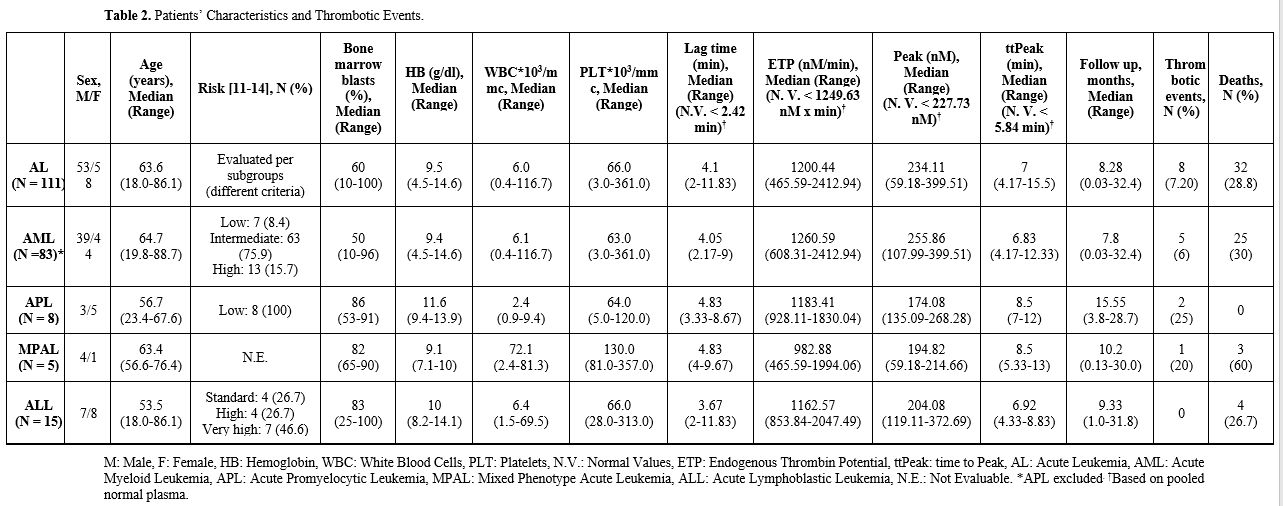 |
Table 2. Patients’ Characteristics and Thrombotic Events. |
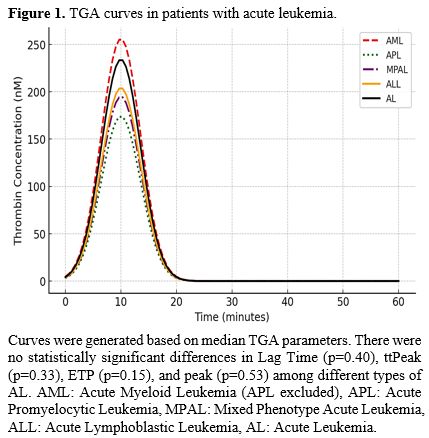 |
Figure 1. TGA curves in patients with acute leukemia. |
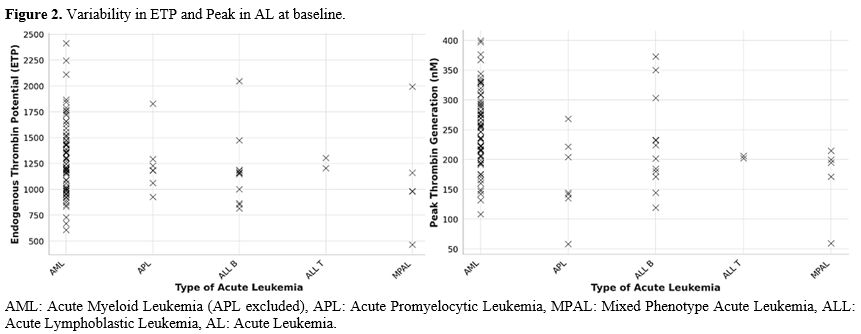 |
Figure 2. Variability in ETP and Peak in AL at baseline. |
One hundred and one patients (90.9%) presented an increase in Lag Time, 74 (66.7%) in ttPeak, 70 (63.1%) in ETP, 58 (52.3%) in peak.
There were no statistically significant differences in Lag Time (p=0.40), ttPeak (p=0.33), ETP (p=0.15), and peak (p=0.53) among different types of AL.
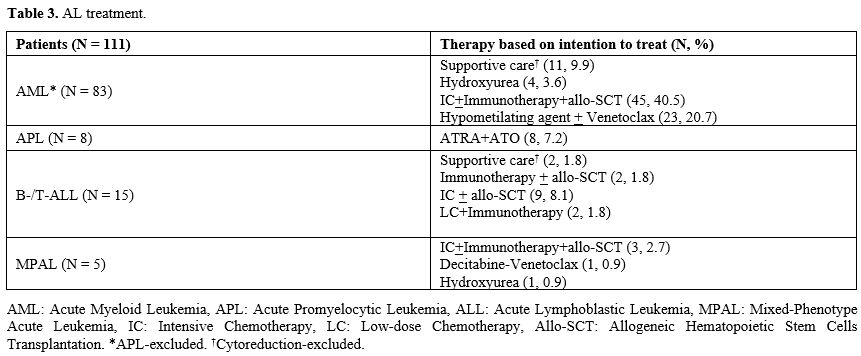
AL treatment is reported in Table 3.
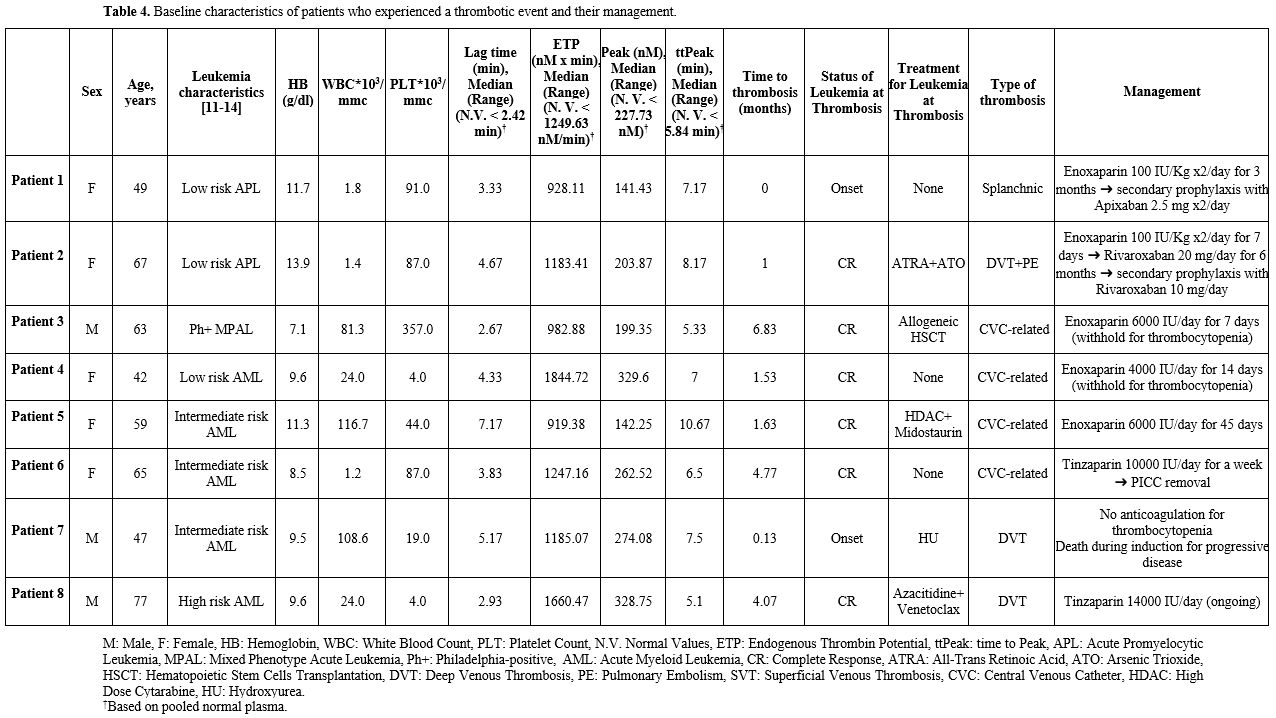
Thrombotic events. Eight patients (7.2%) experienced thrombotic events: 4 (3.6%) deep venous thrombosis (DVT) and 4 (3.6%) CVC-related thrombosis. All eight patients had a CVC at the time of thrombosis. Two patients had a history of previous VTE. Five (4.5%) were diagnosed with AML (APL-excluded), 2 (1.8%) by APL, and 1 (0.9%) by Philadelphia-positive-MAPL. Time from diagnosis to thrombosis was 0.13 months (range 0-4.07).
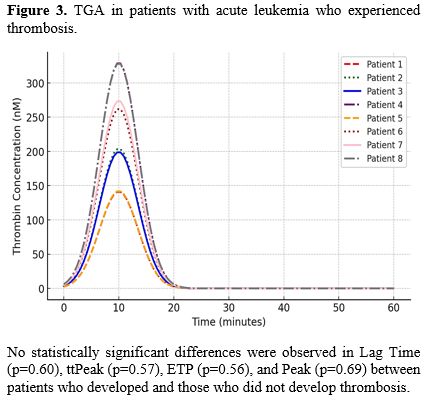
All eight patients presented an increase in Lag Time (100%), 6 in ttPeak (75%), 4 in peak (50%), and 1 (12.5%) in ETP (Figure 3).
No statistically significant differences were observed in Lag Time (p=0.60), ttPeak (p=0.57), ETP (p=0.56), or Peak (p=0.69) between patients who developed thrombosis and those who did not. Similarly, no significant differences in thrombotic events were found across different types of AL (p=0.23) or treatment (p=0.62). Baseline platelet count (p=0.33), LDH (p=0.35), and hematocrit (p=0.96) were also not predictive of thrombosis development. In contrast, a statistically significant association was found between a history of previous VTE and the occurrence of thrombosis (p=0.03).
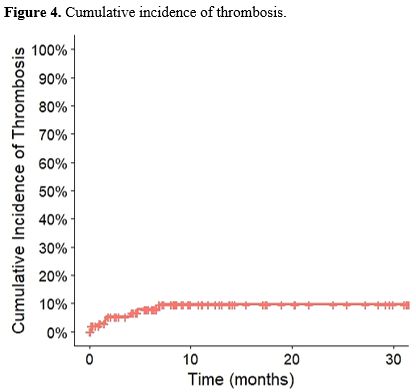
The cumulative incidence of thrombotic events is shown in Figure 4.
Management of thrombotic events. Two thrombotic events occurred at the onset of acute leukemia, while six events developed in patients who were in complete remission.
At disease onset, one patient with low-risk acute promyelocytic leukemia (APL) was diagnosed with splanchnic vein thrombosis and was treated with enoxaparin 100 IU/kg twice daily for three months, achieving complete resolution; the patient was subsequently switched to secondary prophylaxis with apixaban 2.5 mg twice daily. Another patient with intermediate-risk acute myeloid leukemia (AML) developed deep vein thrombosis (DVT) of the lower limbs at diagnosis; anticoagulation was not feasible due to severe thrombocytopenia, and the patient died during induction therapy from progressive disease.
All other thrombotic events occurred after patients had achieved complete remission and were managed with low molecular weight heparin (LMWH), specifically enoxaparin and tinzaparin. Four events were catheter-related: one patient received a full 45-day course of enoxaparin at 100 IU/kg/day, while the remaining three received enoxaparin at 100 IU/kg/day or tinzaparin at prophylactic doses for 7–14 days. In three cases, anticoagulation was interrupted due to thrombocytopenia, with the central venous catheter retained as clinically necessary; in one case, anticoagulation was discontinued after CVC removal. The remaining patients developed lower-limb DVT, one of which was complicated by pulmonary embolism. One patient received tinzaparin at a therapeutic dose, with treatment ongoing during the acute phase, whereas another patient received rivaroxaban 20 mg daily for six months, followed by secondary prophylaxis after DVT resolution.
Management of thrombotic events is summarized in Table 4.
Discussion
Thrombotic complications are a frequent and clinically significant problem in patients with AL. These complications arise from a multifactorial pathophysiological environment, shaped by several factors such as underlying malignancy, antineoplastic treatment, infections, and CVC placement.The current guidelines for the prevention and management of hemostatic complications in AL are based on CCAs and platelet count, which guide the implementation of antihemorrhagic strategies and anticoagulant dosing in patients with thrombotic complications.[15] However, these parameters provide only a partial view of the hemostatic balance, leading to a growing interest in GCAs for a more comprehensive hemostatic assessment.
In our prospective observational study, we investigated the potential utility of TGA in predicting thrombotic events in a large monocentric cohort of newly diagnosed AL patients. While over 60% of patients exhibited elevated ETP and more than half had increased Thrombin Peak levels at diagnosis, consistent with a hypercoagulable state, only 7.2% of the cohort developed thrombotic events during follow-up. No statistically significant association was observed between baseline TGA parameters and the occurrence of thrombosis.
This discrepancy between laboratory hypercoagulability and thrombotic complications suggests that TG alone may not be sufficient to predict thrombosis risk in AL. Indeed, hemostasis in leukemia is profoundly influenced by dynamic and interacting factors. The leukemic microenvironment, for example, is known to induce endothelial activation, inflammatory cytokine release, and microparticle formation, which may promote coagulation independently of thrombin kinetics.[16]
We evaluated TGA parameters at baseline. However, as discussed above, hemostatic parameters undergo changes throughout the disease's progression. Betticher et al.[10] reported that TGA parameters at baseline do not predict the development of thrombotic complications in pediatric ALL; however, ETP values at 8-12 days from the start of induction therapy were predictive of thrombotic complications (p < 0.05). Consistently, Rozen et al.[17-18] found that treatment during the induction phase and late intensification in pediatric ALL leads to an increase in ETP and peak levels. Furthermore, native asparaginase leads to a more marked increase in enzyme activity than peg-asparaginase. However, this finding is in contrast with the higher incidence of thrombosis with peg-asparaginase vs native asparaginase during induction (p = 0.0035) and late intensification (p = 0.0096).[19]
These experiences suggest that a dynamic evaluation of TG parameters may be useful for predicting thrombotic complications in the setting of ALL. No data is available on AML. However, Raso et al.[7] failed to demonstrate an association between TG, indirectly calculated with a TEG algorithm at three timepoints (diagnosis, during the first cycle of chemotherapy, at the end of chemotherapy), and thrombotic or hemorrhagic complications.
Another interesting finding in our experience is the consistent increase in Lag Time across our cohort. While it is true that a shortened Lag Time is associated with hypercoagulability,[20] several hypotheses may explain the paradox of an increased Lag Time in a hypercoagulable state. Lag time is prevalently determined by levels of tissue factor pathway inhibitor (TFPI), protein S (PS), factor VII (FVII), FIX, and fibrinogen. Increased levels of TFPI, an endogenous Kunitz-type serine protease inhibitor expressed by endothelial cells and platelets that inhibits both the TF/FVIIa complex and early forms of prothrombinase, could explain an increase in lag time. It is known that in APL there is an increase in TFPI levels, but they seem to be normal in other AMLs and ALLs.[21-24] However, data regarding TFPI levels in AMLs and ALLs is based on small numbers of patients. Another possible explanation could be the binding of the initial traces of thrombin to fibrinogen/fibrin, which hinders the feedback activation of upstream coagulation factors by thrombin itself.[25] In our cohort, the median fibrinogen levels were normal (363 mg/dl). However, we did not evaluate TFPI, PS, FVII, and FIX levels, limiting further analysis. Despite the increased lag time, unquestionably, the higher ETP and Peak values indicate a hypercoagulable state.
Interestingly, all thrombotic events in our cohort occurred in patients with a CVC, reinforcing the established role of CVCs as a risk factor for venous thromboembolism[26-27] and the relevance of non-leukemia-associated factors in the evaluation of thrombotic risk in this setting. Another notable finding was the association between thrombotic events after the diagnosis of AL and a history of VTE (p=0.03). Although our analysis is limited by the small sample size and low number of events, this result is consistent with previous reports in the literature.[28-29]
In our analysis, we also evaluated the possible association between platelet count and thrombotic complications. Martella et al.[28] and Paterno et al.[29] demonstrated an association between a higher platelet count and thrombosis development; however, in our cohort, it was not statistically significant (p=0.33). Nevertheless, we did not assess platelet function, which is not analyzed by TGA. Several studies have highlighted the relevance of leukemia-related thrombocytopenia and thrombocytopathy in the evaluation of hemostatic complications. However, both conditions appear to be associated with hemorrhagic complications, while an association with thrombotic ones seems unlikely.[7,30-33]
The main strength of this manuscript is that it addresses an important and scarcely explored topic, providing insights into coagulation abnormalities and thrombotic events across different types of acute leukemia. The main limitations of this report are the heterogeneity of the study population, both in terms of disease type and treatment, the small number of patients in each subgroup, the small number of thrombotic events, and the limited follow-up. Hemorrhagic events were not systematically recorded, as the study was designed to focus on thrombotic complications.
Conclusions
Our data confirm that thrombin generation is frequently elevated at the onset of acute leukemia. While baseline TGA did not predict thrombotic complications, this finding should not be interpreted as dismissing the clinical utility of TGA. On the contrary, increasing evidence suggests that a dynamic evaluation of TGA parameters throughout key treatment phases (diagnosis, induction, consolidation, remission, and relapse) could be useful in predicting thrombotic and hemorrhagic events in this setting. Moreover, integrating platelet function tests and inflammatory biomarkers could further enhance its predictive and clinical utility in this setting.Contributions
C.A. conceived and designed the study. B.M. wrote the manuscript. M.R. and D.M.S. analyzed the samples. L.A., B.M.L., F.A., and P.A. interpreted the data. B.E. and S.C. critically revised the manuscript. All authors have read and approved the final version of the manuscript and agree to be held responsible for the integrity of the work.Data availability
The data presented in this study are available on request from the corresponding author.Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards.References
- Mulder F.I., Horváth-Puhó E., van Es N., van
Laarhoven H.W.M., Pedersen L., Moik F., Ay C., Büller H.R., Sørensen
H.T. Venous thromboembolism in cancer patients: a population-based
cohort study. Blood. 2021 Apr 8;137(14):1959-1969. https://doi.org/10.1182/blood.2020007338 PMid:33171494
- Moik
F., Ay C., Pabinger I. Risk prediction for cancer-associated thrombosis
in ambulatory patients with cancer: past, present and future. Thromb
Res. 2020 Jul;191 Suppl 1:S3-S11. https://doi.org/10.1016/S0049-3848(20)30389-3 PMid:32736775
- Bakalov
V., Tang A., Yellala A., Kaplan R., Lister J., Sadashiv S. Risk factors
for venous thromboembolism in hospitalized patients with hematological
malignancy: an analysis of the National Inpatient Sample, 2011-2015.
Leuk Lymphoma. 2020 Feb;61(2):370-376. https://doi.org/10.1080/10428194.2019.1666380 PMid:31545108
- Wang
T.F., Leader A., Sanfilippo K.M. Thrombosis and bleeding in
hematological malignancy. Best Pract Res Clin Haematol. 2022
Mar;35(1):101353. https://doi.org/10.1016/j.beha.2022.101353 PMid:36030068
- Bønløkke
S.T., Ommen H.B., Hvas A.M. Altered Fibrinolysis in Hematological
Malignances. Semin Thromb Hemost. 2021 Jul;47(5):569-580. https://doi.org/10.1055/s-0041-1725099 PMid:34058766
- Al-Samkari
H., Connors J.M. Managing the competing risks of thrombosis, bleeding,
and anticoagulation in patients with malignancy. Blood Adv. 2019 Nov
26;3(22):3770-3779. https://doi.org/10.1182/bloodadvances.2019000369 PMid:31770442 PMCid:PMC6880899
- Raso
S., Lucchesi A., Sardo M., Annibali O., Sucato V., Ciaccio M., Vitale
S., Dolce A., Giordano G., Siragusa S., Napolitano M. Global hemostasis
assays in acute myeloid leukemia: results of an observational
prospective study. Blood Transfus. 2024 Jan;22(1):65-74. https://doi.org/10.2450/BloodTransfus.575
- Whiting D., DiNardo J.A. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014 Feb;89(2):228-32. https://doi.org/10.1002/ajh.23599 PMid:24123050
- Depasse
F., Binder N.B., Mueller J., Wissel T., Schwers S., Germer M., Hermes
B., Turecek P.L. Thrombin generation assays are versatile tools in
blood coagulation analysis: A review of technical features, and
applications from research to laboratory routine. J Thromb Haemost.
2021 Dec;19(12):2907-2917. https://doi.org/10.1111/jth.15529 PMid:34525255 PMCid:PMC9291770
- Betticher
C., Bertaggia Calderara D., Matthey-Guirao E., Gomez F.J., Aliotta A.,
Lemmel E., Ceppi F., Alberio L., Rizzi M. Global coagulation assays
detect an early prothrombotic state in children with acute
lymphoblastic leukemia. J Thromb Haemost. 2024 Sep;22(9):2482-2494. https://doi.org/10.1016/j.jtha.2024.05.032 PMid:38897386
- Döhner
H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T.,
Dombret H., Ebert B.L., Fenaux P., Larson R.A., Levine R.L., Lo-Coco
F., Naoe T., Niederwieser D., Ossenkoppele G.J., Sanz M., Sierra J.,
Tallman M.S., Tien H.F., Wei A.H., Löwenberg B., Bloomfield C.D.
Diagnosis and management of AML in adults: 2017 ELN recommendations
from an international expert panel. Blood. 2017 Jan 26;129(4):424-447. https://doi.org/10.1182/blood-016-08-733196 PMid:27895058 PMCid:PMC5291965
- Döhner
H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H.,
Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., Larson R.A.,
Levine R.L., Miyazaki Y., Niederwieser D., Ossenkoppele G., Röllig C.,
Sierra J., Stein E.M., Tallman M.S., Tien H.F., Wang J., Wierzbowska
A., Löwenberg B. Diagnosis and management of AML in adults: 2022
recommendations from an international expert panel on behalf of the
ELN. Blood. 2022 Sep 22;140(12):1345-1377. https://doi.org/10.1182/blood.2022016867 PMid:35797463
- Sanz
M.A., Fenaux P., Tallman M.S., Estey E.H., Löwenberg B., Naoe T.,
Lengfelder E., Döhner H., Burnett A.K., Chen S.J., Mathews V., Iland
H., Rego E., Kantarjian H., Adès L., Avvisati G., Montesinos P.,
Platzbecker U., Ravandi F., Russell N.H., Lo-Coco F. Management of
acute promyelocytic leukemia: updated recommendations from an expert
panel of the European LeukemiaNet. Blood. 2019 Apr
11;133(15):1630-1643. https://doi.org/10.1182/blood-2019-01-894980 PMid:30803991 PMCid:PMC6509567
- Gökbuget
N., Boissel N., Chiaretti S., Dombret H., Doubek M., Fielding A., Foà
R., Giebel S., Hoelzer D., Hunault M., Marks D.I., Martinelli G.,
Ottmann O., Rijneveld A., Rousselot P., Ribera J., Bassan R. Diagnosis,
prognostic factors, and assessment of ALL in adults: 2024 ELN
recommendations from a European expert panel. Blood. 2024 May
9;143(19):1891-1902. https://doi.org/10.1182/blood.2023020794 PMid:38295337
- Wang
T.F., Makar R.S., Antic D., Levy J.H., Douketis J.D., Connors J.M.,
Carrier M., Zwicker J.I. Management of hemostatic complications in
acute leukemia: Guidance from the SSC of the ISTH. J Thromb Haemost.
2020 Dec;18(12):3174-3183. https://doi.org/10.1111/jth.15074 PMid:33433069 PMCid:PMC7909744
- Olivi
M., Di Biase F., Lanzarone G., Arrigo G., Martella F., Apolito V.,
Secreto C., Freilone R., Bruno B., Audisio E., Ferrero D., Beggiato E.,
Cerrano M. Thrombosis in Acute Myeloid Leukemia: Pathogenesis, Risk
Factors and Therapeutic Challenges. Curr Treat Options Oncol. 2023
Jun;24(6):693-710. https://doi.org/10.1007/s11864-023-01089-w PMid:37099265
- Rozen
L., Noubouossie D.F., Dedeken L., Huybrechts S., Le P.Q., Ferster A.,
Demulder A. Thrombin Generation Test During Asparaginase Treatment In
Children With Acute Lymphoblastic Leukemia, Blood, Volume 122, Issue
21, 2013, Page 2367, ISSN 0006-4971, https://doi.org/10.1182/blood.V122.21.2367.2367
- Rozen
L., Noubouossie D., Dedeken L., Huybrechts S., Lê P.Q., Ferster A.,
Demulder A. Different profile of thrombin generation in children with
acute lymphoblastic leukaemia treated with native or pegylated
asparaginase: A cohort study. Pediatr Blood Cancer. 2017
Feb;64(2):294-301. https://doi.org/10.1002/pbc.26228 PMid:27605400
- Chen
R., Atenafu E.G., Seki J., Liu X., Chan S., Gupta V., Maze D., Shuh
A.C., Minden M.D., Yee K., Schimmer A.D., Sibai H. Venous
thromboembolism incidence associated with pegylated asparaginase (ASP)
compared to the native L-ASP: A retrospective analysis with an
ASP-based protocol in adult patients with acute lymphoblastic
leukaemia. Br J Haematol. 2023 May;201(4):645-652. https://doi.org/10.1111/bjh.18683 PMid:36794878
- Al Dieri R., de Laat B., Hemker H.C. Thrombin generation: what have we learned? Blood Rev. 2012 Sep;26(5):197-203. https://doi.org/10.1016/j.blre.2012.06.001 PMid:22762893
- Bassi
S.C., Rego E.M. Tissue Factor Pathway Inhibitor (TFPI) May be Another
Important Factor in the Coagulopathy in Acute Promyelocytic Leukemia
(APL). Blood 2015; 126 (23): 2278. https://doi.org/10.1182/blood.V126.23.2278.2278
- Hambley
B.C., Tomuleasa C., Ghiaur G. Coagulopathy in Acute Promyelocytic
Leukemia: Can We Go Beyond Supportive Care? Front Med (Lausanne). 2021
Aug 17;8:722614. https://doi.org/10.3389/fmed.2021.722614 PMid:34485349 PMCid:PMC8415964
- Iversen
N., Lindahl A.K., Abildgaard U. Elevated plasma levels of the factor
Xa-TFPI complex in cancer patients. Thromb Res. 2002 Jan 1;105(1):33-6.
https://doi.org/10.1016/S0049-3848(01)00404-2 PMid:11864704
- Albayrak
M., Gürsel T., Kaya Z., Koçak U. Alterations in procoagulant,
anticoagulant, and fibrinolytic systems before and after start of
induction chemotherapy in children with acute lymphoblastic leukemia.
Clin Appl Thromb Hemost. 2013 Nov-Dec;19(6):644-51. https://doi.org/10.1177/1076029612450771 PMid:22751908
- Kremers
R.M., Wagenvoord R.J., Hemker H.C. The effect of fibrin(ogen) on
thrombin generation and decay. Thromb Haemost. 2014 Sep
2;112(3):486-94. https://doi.org/10.1160/TH14-02-0172 PMid:24964786
- Verso
M., Agnelli G. Venous thromboembolism associated with long-term use of
central venous catheters in cancer patients. J Clin Oncol. 2003 Oct
1;21(19):3665-75. https://doi.org/10.1200/JCO.2003.08.008 PMid:14512399
- Citla
Sridhar D., Abou-Ismail M.Y., Ahuja S.P. Central venous
catheter-related thrombosis in children and adults. Thromb Res. 2020
Mar;187:103-112. https://doi.org/10.1016/j.thromres.2020.01.017 PMid:31981840
- Martella
F, Cerrano M, Di Cuonzo D, Secreto C, Olivi M, Apolito V, D'Ardia S,
Frairia C, Giai V, Lanzarone G, Urbino I, Freilone R, Giaccone L, Busca
A, Dellacasa CM, Audisio E, Ferrero D, Beggiato E. Frequency and risk
factors for thrombosis in acute myeloid leukemia and high-risk
myelodysplastic syndromes treated with intensive chemotherapy: a two
centers observational study. Ann Hematol. 2022 Apr;101(4):855-867. https://doi.org/10.1007/s00277-022-04770-6 PMid:35128571
- Paterno
G, Palmieri R, Forte V, Del Prete V, Gurnari C, Guarnera L, Mallegni F,
Pascale MR, Buzzatti E, Mezzanotte V, Cerroni I, Savi A, Buccisano F,
Maurillo L, Venditti A, Del Principe MI. Predictors of Early Thrombotic
Events in Adult Patients with Acute Myeloid Leukemia: A Real-World
Experience. Cancers (Basel). 2022 Nov 17;14(22):5640. https://doi.org/10.3390/cancers14225640 PMid:36428732 PMCid:PMC9688263
- Slichter
S.J., Kaufman R.M., Assmann S.F., McCullough J., Triulzi D.J., Strauss
R.G., Gernsheimer T.B., Ness P.M., Brecher M.E., Josephson C.D., Konkle
B.A., Woodson R.D., Ortel T.L., Hillyer C.D., Skerrett D.L., McCrae
K.R., Sloan S.R., Uhl L., George J.N., Aquino V.M., Manno C.S.,
McFarland J.G., Hess J.R., Leissinger C., Granger S. Dose of
prophylactic platelet transfusions and prevention of hemorrhage. N Engl
J Med. 2010 Feb 18;362(7):600-13. https://doi.org/10.1056/NEJMoa0904084 PMid:20164484 PMCid:PMC2951321
- Bao
H.X., Du J., Chen B.Y., Wang Y. The role of thromboelastography in
predicting hemorrhage risk in patients with leukemia. Medicine
(Baltimore). 2018 Mar;97(13):e0137. doi: 10.1097/MD.0000000000010137 https://doi.org/10.1097/MD.0000000000010137 PMid:29595638 PMCid:PMC5895378
- Sabljic
N., Pantic N., Virijevic M., Bukumiric Z., Novakovic T., Pravdic Z.,
Rajic J., Vidovic A., Suvajdzic N., Jaradeh M., Fareed J., Antic D.,
Mitrovic M. Application of Rotational Thromboelastometry in Patients
with Acute Promyelocytic Leukemia. Clin Appl Thromb Hemost. 2022
Jan-Dec;28:10760296221119809. https://doi.org/10.1177/10760296221119809 PMid:35942712 PMCid:PMC9373117
- Zhang L., Liu J., Qin X., Liu W. Platelet-Acute Leukemia Interactions. Clin Chim Acta. 2022 Nov 1;536:29-38. https://doi.org/10.1016/j.cca.2022.09.015 PMid:36122665