Antiretroviral therapy (ART) has transformed the lives of people with HIV (PWH) by suppressing HIV replication, improving cellular immunity, and subsequently reducing AIDS-related morbidity and mortality. Even in the context of broad access to ART, many studies have reported that AIDS-defining opportunistic infections (OIs) and malignancies remain the main reasons for hospitalization and death among PWH, both in high-income[2,3] and in low- and middle-income countries.[4] In addition, the spectrum of opportunistic infections and malignancies has changed over the last decade.[5-7] AIDS-related diseases, especially Pneumocystis jirovecii pneumonia (PCP), have decreased over time, while HIV-related non-AIDS diseases such as cancer and metabolic diseases have increased, also reflecting the aging of PWH.[8,9] Nevertheless, there are a limited number of studies on HIV-related OIs and malignancies among late presenters in China.
Therefore, we investigated the clinical characteristics and distribution of OIs and malignancies, as well as trends in AIDS-related illnesses among PWH in Eastern China.
Methods
Study participants. In this retrospective chart review, data were derived from the medical records of patients hospitalized in the AIDS ward of the First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China, between January 2010 and December 2021.Data collection. Electronic hospitalization data of patients admitted to the AIDS ward were extracted and recorded. Patient characteristics, including age, sex, time of admission, comorbidities (e.g., hypertension, diabetes, hepatitis B and C), time of HIV diagnosis, and CD4+T cell counts, were obtained from clinical records. Participants presenting with the following infections were classified as having opportunistic infections: candida infections (esophageal, intestinal, and candidemia), cryptococcosis (lung, brain, and any other organs), progressive multifocal leukoencephalopathy (PML), PCP, community-acquired pneumonia, pulmonary tuberculosis, extrapulmonary tuberculosis, non-tuberculous mycobacterial (NTM) infection, Talaromyces marneffei infection (lung and sepsis), CMV tissue lesions, toxoplasmosis, and aspergillosis. In addition, we recorded the survival outcome of all participants censored at the time of discharge.
For PWH diagnosed with aggressive B-cell NHL, the following clinical data were further collected: time interval from HIV infection to lymphoma diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status score, pathological subtype information, presence of B symptoms, serum lactate dehydrogenase (LDH) level, Ann Arbor staging, International Prognostic Index (IPI) score, extranodal involvement, bone marrow involvement, and central nervous system involvement. Additionally, the patients' chemotherapy regimens, chemotherapy cycles, and treatment responses were recorded.
Diagnosis and definitions. All participants tested positive for HIV antibodies using the Western blot assay. Opportunistic infections and malignancies were diagnosed based on the 2013 guidelines recommended by the Centers for Disease Control and Prevention in the United States.[10]
PCP typically presents with fever, cough, and progressive dyspnea. Clinical diagnosis is based on characteristic symptoms, chest X-ray showing interstitial infiltrates, or computed tomography (CT) revealing ground-glass opacities. Definitive diagnosis requires pathogen detection in respiratory specimens or bronchoalveolar lavage fluid via staining, immunofluorescence, or molecular methods. Cryptococcal meningitis commonly manifests as headache, fever, nausea, vomiting, cranial nerve damage, altered consciousness, lethargy, and memory impairment. Diagnosis relies on etiological evidence, such as India ink staining. Cryptococcal antigen titers are usually elevated; in disseminated infections, serum cryptococcal antigen is positive. Fungal culture is the gold standard. For PWH, serum cryptococcal antigen testing serves as an initial screening tool. Talaromycosis (caused by Talaromyces marneffei) presents with fever, umbilicated skin rashes, hepatosplenomegaly, lymphadenopathy, and abdominal pain with distension.
Diagnosis relies on etiological or histopathological examination. Toxoplasmic encephalitis commonly presents as focal encephalitis, with headache, confusion, motor deficits, and fever. Clinical diagnosis is supported by typical manifestations, neuroimaging revealing ring-enhancing lesions with edema, or positive Toxoplasma IgG antibodies in serum or tissue fluid. Definitive diagnosis requires pathogen detection in brain biopsy or cerebrospinal fluid (CSF); response to empirical anti-Toxoplasma therapy may provide adjunctive evidence. Tuberculosis diagnosis in PWH, whether pulmonary or extrapulmonary, relies on acid-fast bacilli smear microscopy of sputum, pleural fluid, lymph nodes, or CSF, and GeneXpert MTB/RIF assay. NTM infections are diagnosed by microbiological culture from blood, lymph fluid, bone marrow, or other tissues/body fluids. CMV infection can reactivate, leading to viremia, which is confirmed by polymerase chain reaction (PCR) or antigen detection. CMV colitis diagnosis relies on endoscopy and histopathology, revealing intranuclear and intracytoplasmic inclusion bodies. CMV esophagitis shows large superficial ulcers in the distal esophagus on gastroscopy, with biopsy confirming endothelial CMV inclusions. PML diagnosis integrates clinical features and neuroimaging; PCR detection of JC virus (JCV) DNA in CSF is commonly used, while brain biopsy confirms demyelination and JCV inclusions. Community-acquired pneumonia presents acutely with fever, chills, cough, and dyspnea. Physical signs include focal consolidation; chest X-ray reveals lobar, segmental consolidation, or pleural effusion. Sputum, blood, or pleural fluid culture identifies bacteria; bronchoalveolar lavage PCR detects atypical pathogens. Candidiasis presents with white plaques, pseudomembranes, or erosions on oral or endoscopic examination. Diagnosis is confirmed by culturing Candida species from secretions or observing yeast cells/pseudohyphae microscopically. Aspergillosis chest X-ray shows diffuse, focal, or cavitary infiltrates, halo sign, or air crescent sign. Diagnosis relies on repeated isolation of Aspergillus from respiratory secretions/tissues, with histopathology showing septate hyphae.
All malignancies over the past 12 years were classified as AIDS-defining cancers (ADCs) and non-AIDS-defining cancers (NADCs). Kaposi's sarcoma (KS), invasive B-cell non-Hodgkin lymphoma (NHL), and invasive cervical cancer are ADCs.[11] All other cancers, including lung, liver, breast, prostate, stomach, colorectal, and Hodgkin lymphoma, are NADCs.[12]
Statistical analysis. The R Project for Statistical Computing version 4.2.1 (http://www.r-project.org/) was used as a software environment for statistical computing and graphics. Continuous variables were represented as mean ± standard deviation, and categorical variables as frequencies (percentages). For continuous variables conforming to a normal distribution, the results were compared using Welch's two-sample t-test. Categorical variables were analyzed using Fisher's exact test. Univariate analysis was performed to identify potential predictors for in-hospital mortality in PWH. Candidate variables with a p-value of < 0.05 on univariate analysis were selected for multivariable logistic regression. Statistical significance was considered when the p-value was < 0.05 in all comparisons.
Results
Participant clinical characteristics. A total of 2140 PWH were included in this study, among them 1868 were male (1868/2140, 87.3%) and 272 were female (272/2140, 12.7%). The age distribution was as follows: 930 (930/2140, 43.5%) PWH were 20-39 years old, and 835 (835/2140, 39%) PWH were 40-59 years old. For comorbidity, 12.1% (259/2140) of participants had hypertension, and 6.0% (128/2140) had diabetes. Among all the 2140 PWH, 255 (255/2140, 11.9%) and 39 (39/2140, 1.8%) presented with hepatitis B and C infection, respectively.More than half of participants (1193/2140, 55.7%) had a CD4+T cell count of < 200 cells/μL, and 44.7% (956/2140) were receiving ART at admission. Furthermore, we recorded 174 in-hospital deaths, yielding a mortality rate of 8.1% (174/2140) (Table 1).
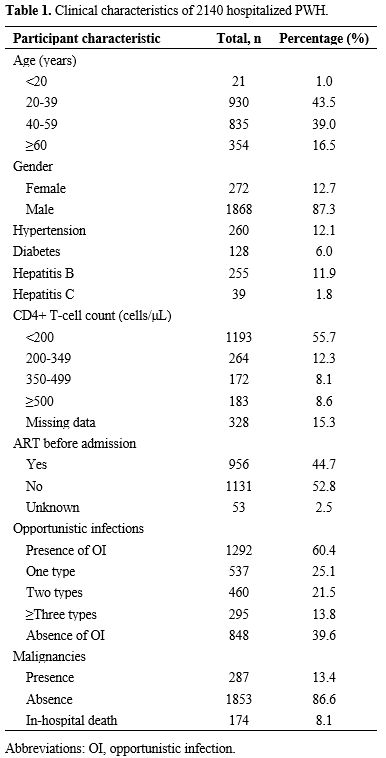
Characteristics of the opportunistic infections and malignancies spectrum. Among the 2140 clinical records, 1292 patients developed opportunistic infections: 537 had one type, and 755 had two or more, resulting in a total of 2536 cases. Community-acquired pneumonia (614/2536, 24.21%) was the most common opportunistic infection, followed by CMV (458/2536, 18.06%), PCP (390/2536, 15.38%), and candidiasis (350/2536, 13.80%) (Figure 1A).
Among the 287 HIV/AIDS patients with malignancies, four presented with two types simultaneously, yielding a total of 291 cases. Of these, 276 tumors were classified into ADCs (n = 103) and NADCs (n = 173). Among the ADCs (Figure 1B), aggressive B-cell NHLs (76/103, 73.79%) were the most common, followed by KS (17/103, 16.50%) and invasive cervical carcinoma (10/103, 9.71%). In NADCs, lung cancer (29/173, 16.76%) was the most common, followed by colorectal cancer (19/173, 10.98%) and liver cancer (13/173, 7.51%). Figure 1C showed a more detailed spectrum of malignancies.
A total of 174 patients died while receiving therapy in our hospital, and the cause of death is shown in Figure 1D. Notably, more than half of these patients died from opportunistic infections, with malignancy being the second leading cause of in-hospital death.
Clinical characteristics and outcomes of AIDS-related aggressive B-Cell NHL. Among the investigated malignancies, AIDS-related lymphoma (ARL) was the most prevalent. Within ADCs, aggressive B-cell NHL predominated, including 51 (51%) diffuse large B-cell lymphoma (DLBCL) cases, 21 (21%) Burkitt lymphoma cases, and 4 (4%) high-grade B-cell lymphoma, not otherwise specified (NOS). Among NADCs, there were 4 (4%) Hodgkin lymphoma cases and 20 (20%) cases of other lymphoma subtypes (e.g., T-cell and immunoblastic lymphoma).
A total of 76 newly diagnosed AIDS-related aggressive B-cell NHL patients were analyzed (Table 2). The median age at diagnosis was 49 years (IQR: 35–59.5). Most patients were male (69/76, 90.79%), and 13.16% (10/76) were unaware of prior HIV infection. HIV duration was <2 years in 57.89% (44/76) and≥2 years in 28.95% (22/76). Non-germinal center B-cell DLBCL was the most common subtype (31/51, 60.78%). CD4+ T-cell counts were <50 cells/uL in 19.74% (15/76), 50–200 cells/uL in 47.37% (36/76), and ≥200 cells/uL in 32.89% (25/76). Elevated LDH was observed in 73.68% (56/76). Ann Arbor stage III/IV was present in 88.16% (67/76), and IPI scores ≥3 were noted in 84.21% (64/76). Extranodal involvement (≥2 sites) occurred in 61.84% (47/76), with central nervous system involvement in 23.68% (18/76). ECOG performance status of 2–4 was observed in 59.21% (45/76), and B symptoms in 52.63% (40/76). Hepatitis B virus coinfection was found in 14.47% (11/76).
Treatment included rituximab-containing regimens in 77.63% (59/76). Chemotherapy was administered to 94.74% (72/76), primarily CHOP (59.72%) or ECHOP (25.00%), with HyperCVAD (12.50%) or high-dose methotrexate (2.78%) in others. More than half of the patients received ≥4 cycles of chemotherapy. One-year overall survival (OS) was 63.16% (48/76), declining to 48.68% (37/76) at 2 years (Table 2).
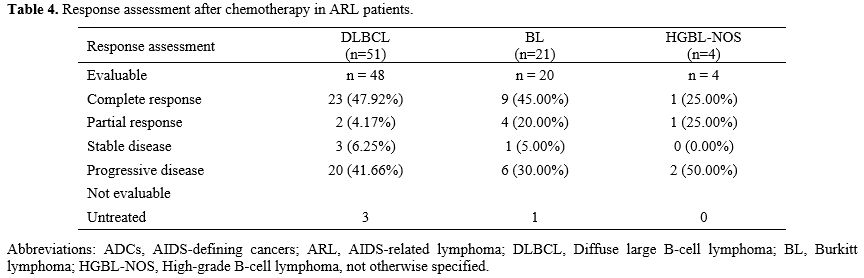
Among 72 treated patients, complete response (CR) occurred in 45.83% (33/72) and partial response (PR) in 9.72% (7/72). For DLBCL (n=48), CR was 47.92% (23/48) and PR 4.17% (2/48); for Burkitt lymphoma (n=20), CR was 45.00% (9/20) and PR 20.00% (4/20) (Table 3). Four patients received no treatment because of financial constraints.
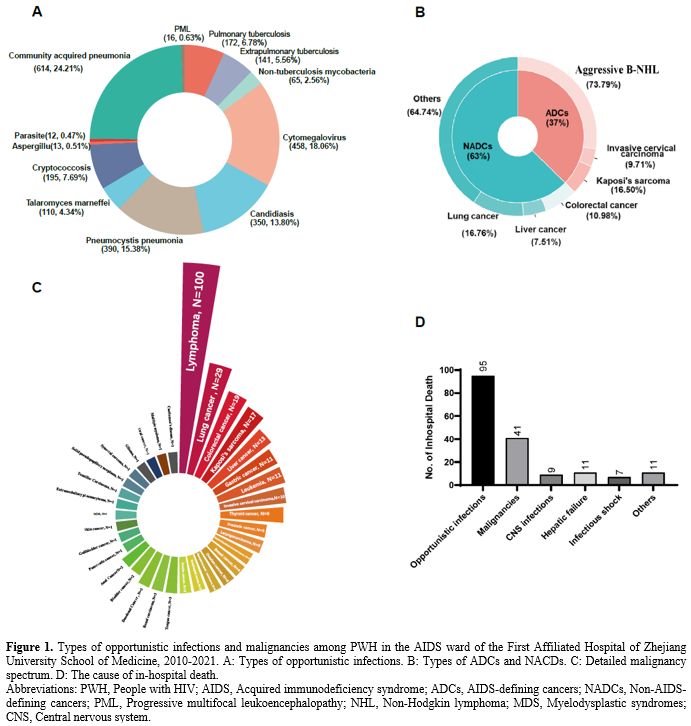
Participant characteristics and disease spectrum changes over time. Upon stratification of hospitalized patients by age, we observed an increase in the proportion of patients aged 50-59 and ≥ 60 years over time (Figure 2A). The proportion of patients with CD4+ T-cell counts <50/ul at admission gradually declined after 2018, except for an increase in 2010. However, the majority of PWH presented with low CD4+ T-cell counts <200/ul (Figure 2B). Over time, the incidence of candidiasis and PCP declined, while the incidence of pulmonary tuberculosis and cryptococcosis remained stable. The incidence of active CMV infection increased over 10 years, then declined during the last two years. (Figure 2C). Conversely, the incidence of Talaromyces marneffei infection gradually increased.
The incidence of malignancies has increased over time, with NADCs occurring more frequently than ADCs. In the 2020-2021 period, the incidence of NADCs became higher than that of OIs (Figure 2C). In-hospital mortality gradually decreased over time. The mortality from cryptococcal meningitis and PCP decreased over time. The in-hospital mortality rate of Talaromyces marneffei infection began to decline after 2018 (Figure 2D).
Identification of independent predictors for PWH in-hospital mortality. Univariate analysis showed that sex, ART usage before admission, arterial hypertension, hepatitis B infection, OIs, malignancies, and CD4+T-cell count were predictors of in-hospital mortality for PWH (p < 0.05). Multivariate logistic regression analysis showed that CD4+T-cell count, malignancies, OIs, and hepatitis B infection were independent predictors for in-hospital mortality (Table 4).
Discussion
This retrospective observational study aimed to identify the disease spectrum among PWH admitted in Eastern China over the past 12 years. We observed that AIDS-related illnesses were the main causes of in-hospital admission and mortality. Most PWH were aged 20-39 years at admission, and 97% were male. More than half of them had a CD4+T-cell count of < 200 cells/μL at admission.Analyzing the OI spectrum, we found that community-acquired pneumonia was the most common, followed by CMV, PCP, and candidiasis. Several studies have reported that community-acquired pneumonia and pulmonary tuberculosis are the most prevalent OIs among PWH.[13-15] In contrast, the incidence of tuberculosis in our study was low. This may be due to non-specific clinical manifestations of tuberculosis in advanced HIV/AIDS patients, where sputum smears were usually negative, as many PWH could not produce high-quality sputum samples.[16,17] These factors likely hindered pulmonary tuberculosis diagnosis in PWH.
A Korean study reported that candidiasis predominates among OIs, while tuberculosis remains a leading OI, partly due to diagnostic challenges in PWH.[15] In Southeast Asian countries like Thailand, endemic infections such as Talaromyces marneffei and extrapulmonary tuberculosis are more prevalent,[18,19] reflecting regional pathogen burdens and lower ART coverage in earlier years. Our lower tuberculosis rates may align with trends in urban Eastern China, where improved screening has reduced incidence compared with rural or high-burden Asian regions.
Previous studies reported that malignancies were a major cause of death among PWH.[20,21] Lung cancer was the most frequent NADC, associated with high smoking habit in China, and NHL was the most common ADC. We observed only 17 cases of KS over the past 12 years, reflecting a low incidence due to the low prevalence of human herpesvirus 8 in China, except in the Northwest region.[22] We noted differences in clinical characteristics between our findings and other reports.[23-25] One possible reason was that, with widespread ART usage, the proportion of ADCs, especially KS, declined over time. Compared with other Asian cohorts, the incidence of NADCs in Japan increased steadily from 2011 to 2021, consistent with our trends, while ADCs decreased due to widespread ART use.[26] Korean studies indicated that NADCs predominated, with lung cancer and hepatocellular carcinoma being most common, linked to high smoking rates and hepatitis prevalence.[27] Indian cohorts reported a higher proportion of NHL and cervical cancer among ADCs, with emerging NADCs like oral and gastrointestinal cancers as survival improved,[28,29] highlighting socioeconomic and healthcare access disparities across Asia.
Patients who died from OIs accounted for more than half of all in-hospital deaths. Among them, most PWH died from respiratory failure due to PCP infection. With the introduction of ART, decreased HIV RNA levels and increased CD4+T cell counts were associated with reduced OIs incidence. Owing to primary prevention and ART, the incidence of acute respiratory failure (ARF) caused by PCP dropped from 70% to 20-40%.[30] A study found that PCP remained the leading cause of ARF in the first decade after the emergence of ART. Nevertheless, in-hospital survival depended on organ dysfunction degree, not HIV-related characteristics.[30]
Our findings have significant implications for clinical practice in Eastern China and similar Asian settings. Persistently high rates of late diagnosis and low CD4 counts at admission underscore the need for expanded HIV screening programs, particularly targeting young males and high-risk groups, to enable earlier ART initiation and prevent OIs. Rising NADCs, especially lung and liver cancers, highlight the importance of integrating cancer prevention strategies, such as smoking cessation counseling, hepatitis vaccination, and routine oncologic screening (e.g., low-dose CT for lung cancer in smokers), into HIV care. Multidisciplinary approaches involving infectious disease specialists, oncologists, and pulmonologists are essential for managing complex cases with overlapping OIs and malignancies, potentially improving in-hospital outcomes and long-term survival. Additionally, these trends support policy efforts to enhance ART accessibility and adherence, reducing morbidity from both ADCs and NADCs across Asia.
Our study has several limitations inherent to its retrospective design, relying on electronic medical records from a single academic center in Eastern China spanning 2010–2021. Detailed individual ART regimens, including specific drug categories or switches, were not systematically captured, as the focus was on hospitalization-related variables such as demographics, comorbidities, laboratory parameters, and outcomes. Retrospective retrieval of these details would depend on potentially incomplete or unavailable historical records, introducing selection bias, missing data, or inaccuracies that could compromise additional analyses. Furthermore, as a hospital-based cohort, our findings may not generalize to community or outpatient settings, potentially overrepresenting severe cases and underestimating milder OIs or early-stage NADCs. We also lacked long-term follow-up data beyond discharge, which limits insights into post-hospitalization outcomes and the causality between variables. These limitations are common in retrospective cohort studies, where granular treatment histories are challenging to reconstruct without prospective elements. Future prospective multicenter studies across Asia could address these gaps to inform regional HIV management better.
Conclusions
Our study provides a comprehensive description of the disease characteristics and changes among PWH in Eastern China over the past 12 years. AIDS-defining illnesses remain the main cause of hospitalization and in-hospital mortality. The proportion of hospitalized patients with severe conditions was high, requiring complex diagnosis and treatment. These characteristics emphasize the importance of hospitals having multidisciplinary team expertise and facilities. There is an urgent need to increase HIV screening with new technologies like home testing, decreasing stigma, and enabling early ART initiation to limit late presentations to care among PWH.Ethical statement and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of the Medical College of Zhejiang University (No. IIT20230188B) and complies with the principles of the Declaration of Helsinki. All data were anonymously collected through the hospital's electronic medical record system. As a retrospective study, the Ethics Committee of the First Affiliated Hospital of the Medical College of Zhejiang University approved waiving informed consent from patients.Data availability
The data will be shared on a reasonable request to the corresponding author.Fundingnding
This study was supported by grants from the National Key R&D Program of China (Nos. 2022YFC2305202, 2021YFC2301900-2021YFC2301901).Authors’ contributions.
B.Z., Y.X. and B.D. conceived and designed the research. Y.X. and B.D. participated in the data collection. Y.X. and D.X. participated in the statistical analysis. Y.X. prepared the original draft. JP.R. and B.D. reviewed and edited the draft. All authors have read and agreed on the published version of the manuscript.References
- Nachega, J.B., et al., Global HIV control: is the glass half empty or half full? Lancet HIV, 2023. https://doi.org/10.1016/S2352-3018(23)00150-9 PMid:37506723
- Luo,
Q., et al., Years of life lost to cancer among the United States HIV
population, 2006-2015. Aids, 2022. 36(9): p. 1279-1286. https://doi.org/10.1097/QAD.0000000000003249 PMid:35608110 PMCid:PMC9283267
- Bielick,
C., et al., National Hospitalization Rates and In-Hospital Mortality
Rates of HIV-Related Opportunistic Infections in the United States,
2011-2018. Clin Infect Dis, 2024. 79(2): p. 487-497. https://doi.org/10.1093/cid/ciae051 PMid:38306316 PMCid:PMC11327786
- Chanie,
E.S., et al., incidence of advanced opportunistic infection and its
predictors among HIV infected children at Debre Tabor referral Hospital
and University of Gondar Compressive specialized hospitals, Northwest
Ethiopia, 2020: A multicenter retrospective follow-up study. Heliyon,
2021. 7(4): p. e06745. https://doi.org/10.1016/j.heliyon.2021.e06745 PMid:33912717 PMCid:PMC8063747
- Buchacz,
K., et al., The HIV Outpatient Study-25 Years of HIV Patient Care and
Epidemiologic Research. Open Forum Infect Dis, 2020. 7(5): p. ofaa123. https://doi.org/10.1093/ofid/ofaa123 PMid:32455145 PMCid:PMC7235508
- Wang,
F., et al., A retrospective study of distribution of HIV associated
malignancies among inpatients from 2007 to 2020 in China. Sci Rep,
2021. 11(1): p. 24353. https://doi.org/10.1038/s41598-021-03672-3 PMid:34934097 PMCid:PMC8692320
- Mathoma,
A., B. Sartorius, and S. Mahomed, The Trends and Risk Factors of
AIDS-Defining Cancers and Non-AIDS-Defining Cancers in Adults Living
with and without HIV: A Narrative Review. J Cancer Epidemiol, 2024.
2024: p. 7588928. https://doi.org/10.1155/2024/7588928 PMid:38549952 PMCid:PMC10978085
- Schlabe,
S., et al., People living with HIV, HCV and HIV/HCV coinfection in
intensive care in a German tertiary referral center 2014-2019.
Infection, 2023. https://doi.org/10.1007/s15010-023-02032-9 PMid:37055704
- Butterfield,
T.R., A.L. Landay, and J.J. Anzinger, Dysfunctional Immunometabolism in
HIV Infection: Contributing Factors and Implications for Age-Related
Comorbid Diseases. Curr HIV/AIDS Rep, 2020. 17(2): p. 125-137. https://doi.org/10.1007/s11904-020-00484-4 PMid:32140979 PMCid:PMC8760627
- Siberry,
G.K., et al., Guidelines for the prevention and treatment of
opportunistic infections in HIV-exposed and HIV-infected children:
recommendations from the National Institutes of Health, Centers for
Disease Control and Prevention, the HIV Medicine Association of the
Infectious Diseases Society of America, the Pediatric Infectious
Diseases Society, and the American Academy of Pediatrics. Pediatr
Infect Dis J, 2013. 32 Suppl 2(0 2): p. i-KK4. https://doi.org/10.1093/jpids/pit074 PMid:26619492 PMCid:PMC6281050
- Yarchoan, R. and T.S. Uldrick, HIV-Associated Cancers and Related Diseases. N Engl J Med, 2018. 378(11): p. 1029-1041. https://doi.org/10.1056/NEJMra1615896 PMid:29539283 PMCid:PMC6890231
- Dlamini, Z., et al., HIV-Associated Cancer Biomarkers: A Requirement for Early Diagnosis. Int J Mol Sci, 2021. 22(15). https://doi.org/10.3390/ijms22158127 PMid:34360891 PMCid:PMC8348540
- Meng,
S., et al., spectrum and mortality of opportunistic infections among
HIV/AIDS patients in southwestern China. Eur J Clin Microbiol Infect
Dis, 2023. 42(1): p. 113-120. https://doi.org/10.1007/s10096-022-04528-y PMid:36413338 PMCid:PMC9816182
- Woldegeorgis,
B.Z., et al., incidence and predictors of opportunistic infections in
adolescents and adults after the initiation of antiretroviral therapy:
A 10-year retrospective cohort study in Ethiopia. Front Public Health,
2022. 10: p. 1064859. https://doi.org/10.3389/fpubh.2022.1064859 PMid:36589962 PMCid:PMC9797664
- Kim,
Y.J., et al., Opportunistic diseases among HIV-infected patients: a
multicenter-nationwide Korean HIV/AIDS cohort study, 2006 to 2013.
Korean J Intern Med, 2016. 31(5): p. 953-60. https://doi.org/10.3904/kjim.2014.322 PMid:27117317 PMCid:PMC5016273
- Faria,
M., et al., Effectiveness of GeneXpert® in the diagnosis of
tuberculosis in people living with HIV/AIDS. Rev Saude Publica, 2021.
55: p. 89.
- Wilson,
D., P. Cudahy, and P.K. Drain, Urine and sputum tuberculosis tests:
defining the trade-offs in endemic HIV and tuberculosis settings.
Lancet Glob Health, 2023. 11(6): p. e809-e810. https://doi.org/10.1016/S2214-109X(23)00215-2 PMid:37202010
- Chaicharoen,
H., et al., Clinical characteristics and mortality of tuberculosis
among adults living with HIV/AIDS: A single center, retrospective
cohort study in Thailand. Int J STD AIDS, 2025. 36(1): p. 56-64. https://doi.org/10.1177/09564624241289986 PMid:39361818
- Qin,
Y., et al., Burden of Talaromyces marneffei infection in people living
with HIV/AIDS in Asia during ART era: a systematic review and
meta-analysis. BMC Infect Dis, 2020. 20(1): p. 551. https://doi.org/10.1186/s12879-020-05260-8 PMid:32727383 PMCid:PMC7392840
- Galati, D. and S. Zanotta, The Role of Cancer Biomarkers in HIV Infected Hosts. Curr Med Chem, 2016. 23(22): p. 2333-49. https://doi.org/10.2174/0929867323666160530145102 PMid:27237819
- Omar,
A., N. Marques, and N. Crawford, Cancer and HIV: The Molecular
Mechanisms of the Deadly Duo. Cancers (Basel), 2024. 16(3). https://doi.org/10.3390/cancers16030546 PMid:38339297 PMCid:PMC10854577
- Zhang,
J., et al., Association between human herpesvirus 8 and lipid profile
in northwest China: A cross-sectional study. J Med Virol, 2024. 96(8):
p. e29794. https://doi.org/10.1002/jmv.29794 PMid:39101375
- Robbins, H.A., et al., Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst, 2015. 107(4). https://doi.org/10.1093/jnci/dju503
- Highly
active antiretroviral therapy and incidence of cancer in human
immunodeficiency virus-infected adults. J Natl Cancer Inst, 2000.
92(22): p. 1823-30. https://doi.org/10.1093/jnci/92.22.1823 PMid:11078759
- Castilho, J.L., et al., CD4/CD8 Ratio and Cancer Risk Among Adults With HIV. J Natl Cancer Inst, 2022. 114(6): p. 854-862. https://doi.org/10.1093/jnci/djac053 PMid:35292820 PMCid:PMC9194634
- Ando,
K., et al., Impact of HIV status on prognosis of malignancies among
people living with HIV in Japan. Cancer, 2024. 130(18): p. 3180-3187. https://doi.org/10.1002/cncr.35351 PMid:38718047
- Lee,
S.O., et al., Changing trends in the incidence and spectrum of cancers
between 1990 and 2021 among HIV-infected patients in Busan, Korea. J
Infect Chemother, 2023. 29(6): p. 571-575. https://doi.org/10.1016/j.jiac.2023.01.018 PMid:36716862
- Chatterji,
S., et al., Pneumocystis jirovecii Pneumonia in Cancer Patients, a
Lethal Yet Fully Preventable Disease: Insights From a Tertiary Cancer
Center in East India. Asia Pac J Clin Oncol, 2025. 21(3): p. 319-327. https://doi.org/10.1111/ajco.14156 PMid:39953677 PMCid:PMC12033038
- Das,
S., et al., Integrating Cervical Cancer Screening and Human
Immunodeficiency Virus Care among Women Living with Human
Immunodeficiency Virus: A Call for Action. Indian J Public Health,
2025. 69(Suppl 1): p. S71-s74. https://doi.org/10.4103/ijph.ijph_1100_24 PMid:40898800
- Barbier, F., et al., Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med, 2009. 35(10): p. 1678-86. https://doi.org/10.1007/s00134-009-1559-4 PMid:19575179 PMCid:PMC7094937