Liver needle biopsy is still regarded as the "gold standard" method for the assessment of liver fibrosis. However, due to its invasive nature, this procedure carries a risk of various complications and, by sampling only a limited portion of the liver, may fail to represent the entire parenchyma fully. For these reasons, there has been a growing interest in alternative non-invasive methods, and, in recent years, the number of studies investigating non-invasive approaches for evaluating liver fibrosis has increased substantially.[4-7]
The mechanism of liver fibrosis development is based on the differentiation of inflammatory monocytes into macrophages, which subsequently activate hepatic stellate cells.[7] Interleukin-34 (IL-34) plays a role in macrophage differentiation through the macrophage colony-stimulating factor receptor (M-CSF or CD115+). Several studies have suggested that serum IL-34 may contribute to the development of fibrosis.[8]
Fibrosis in the liver increases tissue stiffness and reduces elasticity. These biomechanical changes can be evaluated using ultrasound-based shear wave elastography (SWE). SWE mathematically quantifies tissue stiffness by measuring the horizontal displacement induced by acoustic waves within the tissue; the shear wave velocity is directly proportional to tissue stiffness and is expressed in meters per second (m/s) or kilopascals (kPa).[9-11] As a non-invasive method, SWE is widely used for assessing liver fibrosis. Supersonic shear imaging-based SWE offers several advantages over transient elastography (TE): it can be integrated with a conventional ultrasound probe to allow routine ultrasonographic examination, enables precise localization of the measurement area using B-mode ultrasound, is not limited by the presence of ascites, and provides image acquisition at a higher frame rate.[12,13]
The European Association for the Study of the Liver (EASL) guidelines emphasize the need for non-invasive tests, rather than invasive biopsies, particularly in patients with HBV DNA levels greater than 2000 IU/mL and normal alanine aminotransferase (ALT) levels.[14,15] In this context, research focusing on liver imaging techniques and serum biomarkers has gained prominence.
A total of 392 treatment-naive patients diagnosed with CHB and evaluated for liver biopsy indication were initially assessed for this prospective study, of whom 105 patients were included after applying the exclusion criteria. The primary aim of our study was to compare non-invasive diagnostic tests (SWE and serum IL-34) with liver needle biopsy pathology results, simultaneously assessing liver fibrosis. In this manner, the study aimed to assess the reliability of non-invasive tests in evaluating liver fibrosis.
Materials and Methods
Patient selection. Between June 10, 2021, and May 31, 2022, a total of 392 treatment-naive patients diagnosed with CHB who presented to the Infectious Diseases and Clinical Microbiology outpatient clinic at University of Health Sciences, Istanbul Haseki Training and Research Hospital were evaluated for liver biopsy indication. After applying exclusion criteria, 105 eligible patients were included in this prospective study.HCV/HIV co-infected patients, patients with compensated/decompensated liver cirrhosis, alcoholic liver disease, non-alcoholic fatty liver disease, autoimmune liver disease, chronic liver disease induced by other causes, chronic kidney failure, patients who were pregnant or morbidly obese (BMI>40; body mass index), and patients who provided inadequate liver needle biopsy specimen or refused liver needle biopsy, were excluded.
Ethics committee approval was obtained from the University of Health Sciences, İstanbul Haseki Training and Research Hospital, Clinical Research Ethics Committee, dated June 9, 2021, and decision number 34-2021, in accordance with the Declaration of Helsinki.
Patients' age, gender, weight, height, body mass index, alcohol use, hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), antibodies against HBeAg (Anti-HBe), HBV DNA, delta antibody, Anti-HCV, Anti-HIV, serum ALT, serum aspartate aminotransferase (AST), total bilirubin, serum urea, serum creatinine, estimated glomerular filtration rate (eGFR), alpha feto protein (AFP), and platelet count were recorded, before liver needle biopsy, to Microsoft Office Excell Professional ® program and IBM SPSS V23 statistical program.
Measurement of cytokines (serum IL-34). Before the liver needle biopsy procedure, 10 mL blood samples from all patients were collected into a serum separator tube and centrifuged at 4000 rpm for 10 minutes to separate the serum. Serum samples were stored at -80 degrees. At the end of the study, serum samples were allowed to reach room temperature in accordance with the manufacturer's instructions and measured using the sandwich enzyme-linked immunosorbent assay (ELISA) method with the Cloud-Clone Corp. (SEC007Hu 96 Tests) Enzyme-linked Immunosorbent Assay Kit for IL-34, specific for Homo sapiens.
Measurement of liver stiffness by shear wave elastography. SWE measurements were performed before the liver needle biopsy procedure, after at least six hours of fasting to minimize the effect of portal vein flow, using the SWE technique with a Mindray Resona 7 device (China) and an SC 6-1 U convex probe (frequency range: 1.3–6 MHz). SWE measurements were independently conducted by two different experienced radiologists, each with five years of SWE experience, to minimize operator-dependent variability. Inter-observer reproducibility was assessed using the intraclass correlation coefficient (ICC). An ICC value ≥0.75 was considered indicative of excellent agreement. Patients with incidental liver mass or portal vein thrombus were excluded. On a grayscale image captured from the right lobe of the liver while the patient is in the supine position and holding their breath for a few seconds, a ~2×3 cm sample box was placed with an intercostal approach. At least three regions of interest (ROIs) (1 cm2) were placed with their centers at least 2–5 cm below the Glisson capsule to avoid reverberation artifacts or subcapsular stiffness, in line with the recommendations of the European Federation of Societies for Ultrasound in Medicine and Biology and the World Ultrasound Federation. At least 3 ROIs were placed in each sample box, and their mean values were calculated. At least five sample boxes were created for each patient. The mean of the mean liver stiffness measurement values measured from all sample boxes was recorded. Liver stiffness was expressed in kPa.
Liver biopsy procedure and staging of fibrosis. Needle biopsy materials of the patients were obtained from the right lobe of the liver by an experienced radiologist with an 18-gauge Tru-cut needle under the guidance of a US device. All liver biopsy specimens were subjected to the paraffin-embedding process by the University of Health Sciences, İstanbul Haseki Training and Research Hospital, Department of Pathology, and stained with one slide of hematoxylin and eosin and 5 slides of Periodic acid Schiff (PAS), PAS-D, Mason Trichrome, Reticulin (by Silver Impregnation method), and Perls for iron deposition. Fibrosis and histological activity index (HAI) of liver tissue samples were calculated using the Knodell scoring system, modified by İshak, by two different pathologists with at least 10 years of experience, who were unaware of the patients' clinical history. Liver biopsy specimens with at least six portal areas were included in the study. The data obtained from the two pathologists were averaged to facilitate statistical analysis. HAI scores ranged from 0 to 18. Those with HAI<6 were considered the group with mild inflammation, and those with HAI≥6 were considered the patient group with moderate-to-severe inflammation. The fibrosis was graded using the silver impregnation method, based on the density of the reticular fibers. The grade of fibrosis was scored on a scale of 0 to 6. Those with fibrosis 0 and 1 were considered the mild fibrosis group, and those with fibrosis≥2 were considered the moderate-advanced fibrosis group.
Statistical analysis. Data were analyzed using IBM SPSS Version 23. Conformity to normal distribution was evaluated by the Shapiro-Wilk and Kolmogorov-Smirnov tests. An independent two-sample t-test was used to compare normally distributed data according to pairwise groups, and a Mann-Whitney U test was used to compare non-normally distributed data. The chi-square test and Yates correction were used to compare categorical variables across groups. Binary logistic regression analysis was used to examine risk factors affecting fibrosis and HAI. Linear regression analysis was employed to investigate the factors influencing SWE and IL-34 values. Spearman's rho correlation coefficient was used to examine the relationship between the non-normally distributed data. Analysis results were presented as mean ± s. deviation and median (minimum – maximum) for quantitative data, and frequency (percent) for categorical data. The statistical significance level was accepted as p< 0.05.
Results
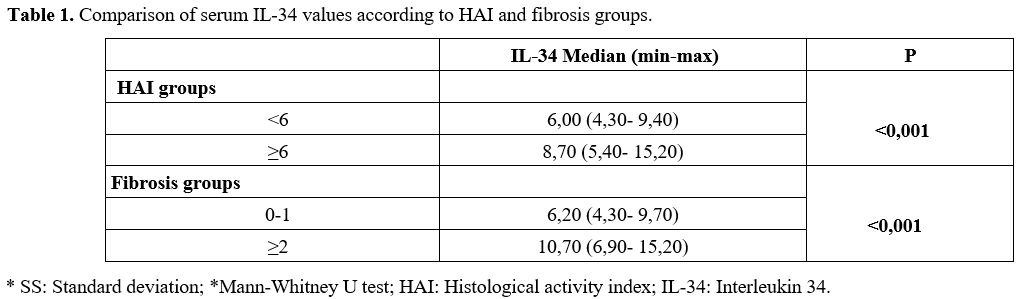
Of the patients, 58 (55%) were male, 47 (45%) were female, and the mean age was 42.97 ± 10.99 years. According to liver needle biopsy histopathological examination, the median serum IL-34 value of patients with a HAI value of <6 was 6.00 pg/ml. In comparison, the median serum IL-34 value of patients with HAI ≥6 was 8.70 pg/ml. A significant difference was found between the median serum IL-34 values of HAI groups (p<0.001). While the median serum IL-34 value in the patient group with a fibrosis value of 0-1 was 6.20 pg/ml, it was 10.70 pg/ml in that with a fibrosis value of ≥2. With respect to the fibrosis groups, a significant difference was found between the median values of serum IL-34 (p<0.001) (Table 1).Inter-radiologist agreement was calculated using the Intraclass correlation coefficient (ICC). A two-way random effects model with absolute agreement (ICC (2,1)) was used in the calculation. SWE measurements showed excellent inter-observer agreement with an ICC of 0.99 (95% CI: 0.97-0.999, p<0.001), indicating high reproducibility between the two radiologists. A statistically significant difference was found between the SWE measurement means in patients with BMI <25 (p=0.030) according to the fibrosis groups. The mean of the group with fibrosis value 0-1 was 6.77 kPa, while the mean of the group with fibrosis value ≥2 was 9.50 kPa. A statistically significant difference was found between the SWE means in patients with a BMI between 25 and 30 (p<0.001) according to the fibrosis groups. There was no statistically significant difference in the SWE means between patients with a BMI ≥30, categorized by fibrosis groups (p = 0.328) (Table 2).
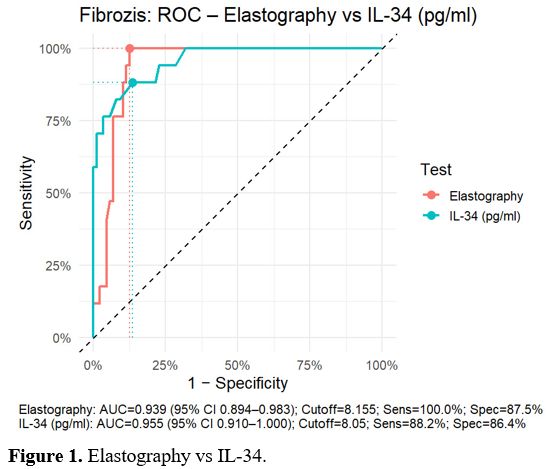
The AUC value obtained from the SWE value was 0.939, resulting in a fibrosis score≥2, which was statistically significant (p<0.001). When the cut-off value was set at 8.18 kPa, the sensitivity was 100%, the specificity was 87.50%, the positive predictive value (PPV) was 60.71%, and the negative predictive value (NPV) was 100%. Moreover, the AUC value of the serum IL-34 level was 0.955, indicating a statistically significant result for fibrosis ≥2 (p < 0.001). When the cut-off value was taken as 8.1 pg/ml, the sensitivity was 88.24%, specificity 86.36%, PPV 55.56%, and NPV 97.44% (Figure 1).
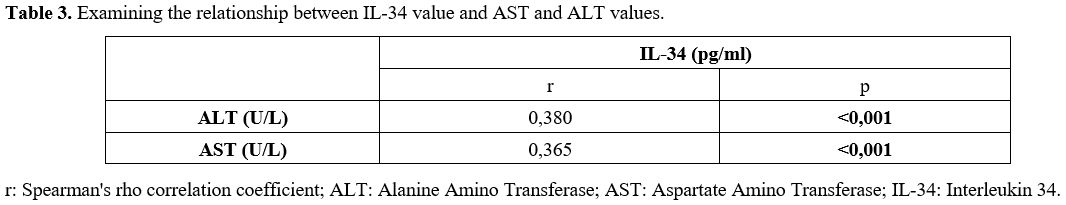
A statistically significant, weak positive correlation was found between serum IL-34 levels and ALT and AST levels (p < 0.001) (Table 3).
Risk factors affecting fibrosis were analyzed by binary logistic regression analysis, including univariate and multivariate models. When the results of the univariate and multivariate models were examined, the risk of fibrosis in female patients was found to be lower than that of male patients (OR=0.214; p=0.022) (OR=0.189; p=0.031). The risk of fibrosis in patients with HBV-DNA results greater than 20,000 IU/mL was found to be 6.474 times higher than in those with 2,000-20,000 IU/mL (p = 0.006). Other criteria assessed were not found to be risk factors (p> 0.050) (Table 4).
Discussion
In this prospective study, serum IL-34 levels and SWE measurements were evaluated in treatment-naive patients with chronic hepatitis B by comparison with liver biopsy findings, including the HAI and fibrosis stage. Our results demonstrate that both IL-34 and SWE exhibit high diagnostic performance in predicting advanced fibrosis (≥F2) and significant necroinflammatory activity (HAI ≥6).In our study, serum IL-34 levels were significantly associated with both the HAI and fibrosis stage. The median IL-34 level was 8.70 pg/mL in patients with HAI ≥6, compared to 6.00 pg/mL in those with HAI <6 (p<0.001). Similarly, patients with fibrosis stage ≥2 had a median IL-34 level of 10.70 pg/mL, whereas those with fibrosis stage 0–1 had a median level of 6.20 pg/mL (p<0.001). These findings suggest that IL-34 may serve as a biomarker reflecting both fibrotic progression and necroinflammatory activity. Wang et al. reported that serum IL-34 levels increased significantly with fibrosis stage in patients with chronic HBV infection, contributing to fibrosis progression through monocyte-macrophage activation, inflammatory cytokine release, and hepatic stellate cell activation.[8] Our study is consistent with these findings, further demonstrating the strong association between IL-34 and histopathological fibrosis scores. In another study conducted in patients with HBV-related hepatocellular carcinoma (HCC), intrahepatic IL-34 levels were found to be significantly elevated, with IL-34 predominantly localized within the cytoplasm of HCC hepatocytes.[16] Similarly, Shoji et al. reported a positive correlation between serum IL-34 levels and the severity of fibrosis in patients with non-alcoholic fatty liver disease (NAFLD), suggesting that IL-34 could serve as an additional biochemical marker for staging liver fibrosis.[17] Preisser et al. reported that IL-34 levels increased significantly with disease progression in HCV-related liver fibrosis.[18] This association has been validated in studies across various etiologies, including HBV, HCV, and NAFLD. Therefore, IL-34 can be regarded not merely as an indirect indicator of tissue injury but also as a dynamic biomarker reflecting the underlying pathophysiological processes of hepatic fibrogenesis and inflammation. In clinical practice, particularly in cases where elastography measurements are compromised by obesity or when existing biomarkers yield conflicting results, IL-34 may serve as a complementary biomarker that enhances diagnostic accuracy and reduces the need for biopsy. Nevertheless, before IL-34 can be adopted into routine clinical practice, methodological standardization, validation in large multicenter prospective cohorts, and the establishment of universally accepted cut-off values are essential.
Compared with established non-invasive biomarkers such as the AST to Platelet Ratio Index (APRI), Fibrosis-4 (FIB-4) score, and Enhanced Liver Fibrosis (ELF) test, which indirectly estimate fibrosis through liver enzyme levels or extracellular matrix components, IL-34 more directly reflects immune-mediated inflammatory and fibrogenic activity. In clinical practice, particularly when elastography is limited by obesity or when existing biomarkers yield inconclusive results, IL-34 may serve as a complementary marker to improve diagnostic accuracy and reduce the need for biopsy. However, before IL-34 can be implemented in routine practice, methodological standardization, validation in large multicenter prospective cohorts, and the definition of universally accepted cut-off values are required.
Castera et al. reported that the SWE technique can only be applied in specialized centers and shows an approximately 20% failure rate in obese individuals due to the influence of subcutaneous adipose tissue on the measurement technique.[19] In our study, considering obesity as a potential confounding factor for SWE measurements, patients were stratified into three groups based on BMI. In the group with BMI <25 kg/m², a significant difference in SWE values was observed between fibrosis stages: patients with fibrosis stage 0–1 had a mean SWE value of 6.77 kPa, whereas those with fibrosis ≥2 had 9.50 kPa (p=0.030). Similarly, in the BMI 25–30 kg/m² group, significant differences were observed between fibrosis stages (p< 0.001). However, in patients with BMI ≥30 kg/m², no significant difference was found between fibrosis stages (p=0.328), and SWE tended to overestimate the fibrosis stage.
These results suggest that obesity may compromise the diagnostic accuracy of SWE. Previous studies have shown that increased BMI and greater skin-to-liver distance reduce measurement reliability and increase variability.[11,20,21] The reduced performance in our obese cohort likely stems from technical limitations: excessive subcutaneous adipose tissue increases the skin-to-liver distance, disrupts the acoustic window, and attenuates shear wave propagation. Heterogeneous fat distribution within the abdominal wall may further cause attenuation and scattering, leading to overestimation or variability in stiffness measurements. Collectively, these factors impair image quality and measurement reliability, suggesting that in clinical practice, SWE alone may be insufficient in obese patients and that combining SWE with serum biomarkers could provide a more reliable approach.
In our study, we also evaluated the diagnostic performance of serum IL-34 and SWE measurements for staging liver fibrosis. ROC analysis determined that the cut-off value of IL-34 was 8.1 pg/mL for fibrosis ≥2 and 7.3 pg/mL for HAI ≥6. In the study by Wang et al., serum IL-34 levels ≥15.83 pg/mL in patients with chronic hepatitis B were associated with the diagnosis of severe fibrosis (F3–F4) with a sensitivity of 86.6% and specificity of 78.7%.[8] These results support the potential use of IL-34 as a biomarker for assessing liver fibrosis. However, the higher cut-off value for advanced fibrosis (F3–F4) reported by Wang et al. highlights that IL-34 levels may vary according to fibrosis stage, emphasizing the need to consider stage-specific cut-off values in clinical interpretation.
For SWE, ROC analysis yielded a cut-off value of 8.18 kPa for predicting fibrosis ≥2. The SWE cut-off values obtained in our study are consistent with recent reports in the literature and are clinically meaningful. These findings largely align with a meta-analysis that included 2,623 CHB patients, which reported a mean threshold of 7.91 kPa, with 88% sensitivity, 83% specificity, and an area under the receiver operating characteristic curve (AUROC) of 0.92. Similarly, in a study by Zhuang et al. involving 539 CHB patients, cut-off values of 7.6 kPa for F2, 9.2 kPa for F3, and 10.4 kPa for F4 were reported, with corresponding sensitivities and specificities of 97%, 96%, and 98%, respectively.[23] These results demonstrate that SWE exhibits high accuracy in diagnosing both early and advanced stages of fibrosis, highlighting its reliability as a non-invasive method. The consistency of the cut-off values obtained in our study with those reported in the literature supports the use of SWE as a reliable tool for staging fibrosis in CHB patients, providing clinically applicable threshold values that may show minor variations according to the local population.
Serum ALT levels are known to be associated with the degree of hepatic inflammation. In the study by Wang et al., IL-34 levels in patients with chronic HBV were reported to be significantly higher in those with elevated aminotransferase levels compared to those with normal aminotransferase levels.[8] Kim WR et al. reported that patients with elevated ALT levels exhibited more severe liver inflammation compared to those with normal ALT levels; however, the correlation between ALT and fibrosis stage was weaker.[24] In our study, serum IL-34 levels showed a statistically significant but weak positive correlation with ALT and AST. This weak correlation supports the potential use of serum IL-34 as a marker of inflammation in patients with elevated ALT levels.
This study provides several strengths, including the simultaneous evaluation of histological and non-invasive assessments in treatment-naive CHB patients, subgroup analyses based on BMI, and determination of ROC-based cut-off values for both IL-34 and SWE, thereby contributing robustly to the current literature. However, there are some limitations to consider. The study was conducted at a single center, which may limit the generalizability of the findings. In addition, the relatively small number of patients with advanced fibrosis (F3–F4) may reduce the precision of diagnostic performance estimates and limit the applicability of our results to broader populations with severe fibrosis.
Conclusions
This study demonstrates that serum IL-34 levels and SWE measurements are reliable non-invasive methods for assessing liver fibrosis in treatment-naive CHB patients. IL-34 showed a strong association with both fibrosis stage and necroinflammatory activity, whereas SWE accurately reflected fibrosis, particularly in non-obese patients. The limited performance of SWE in obese patients highlights the importance of combining it with biomarkers. The combined assessment of IL-34 and SWE may offer a potential approach to reduce the need for liver biopsy.Financial Disclosure
A budget of 400 USD was provided to this study by the Health Sciences University, Sultangazi Haseki Training and Research Hospital, Medical Specialization Ethics Committee, dated January 11, 2021, and protocol number 26.References
- Hsu YC, Huang DQ, Nguyen MH. Global burden of
hepatitis B virus: current status, missed opportunities and a call for
action. Nat Rev Gastroenterol Hepatol. 2023 Aug;20(8):524-537. https://doi.org/10.1038/s41575-023-00760-9 PMid:37024566
- Li
J, Lin Y, Wang X, Lu M. Interconnection of cellular autophagy and
endosomal vesicle trafficking and its role in hepatitis B virus
replication and release. Virol Sin. 2024 Feb;39(1):24-30. doi:
10.1016/j.virs.2024.01.001. Epub 2024 January 9. https://doi.org/10.1016/j.virs.2024.01.001 PMid:38211880 PMCid:PMC10877419
- WHO, Global progress report on Hepatitis B, June 24, 2022, https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Bera
C, Hamdan-Perez N, Patel K. Non-invasive Assessment of Liver Fibrosis
in Hepatitis B Patients. Journal of Clinical Medicine. 2024;
13(4):1046. https://doi.org/10.3390/jcm13041046 PMid:38398358 PMCid:PMC10889471
- Lai
JC, Liang LY, Wong GL. Non-invasive tests for liver fibrosis in 2024:
are there different scales for different diseases? Gastroenterol Rep
(Oxf). 2024 April 11;12:goae024. doi: 10.1093/gastro/goae024. Erratum
in: Gastroenterol Rep (Oxf). 2024 October 12;12:goae096. https://doi.org/10.1093/gastro/goae024 PMid:38605932 PMCid:PMC11009030
- Huang
R, Wu C. Non-invasive tests for assessing liver fibrosis and cirrhosis
in chronic hepatitis B. Lancet Gastroenterol Hepatol. 2025
Apr;10(4):280-282. https://doi.org/10.1016/S2468-1253(25)00009-3 PMid:39983747
- Charoenchue
P, Khorana J, Chitapanarux T, Inmutto N, Na Chiangmai W et al.
Two-Dimensional Shear-Wave Elastography: Accuracy in Liver Fibrosis
Staging Using Magnetic Resonance Elastography as the Reference
Standard. Diagnostics (Basel). 2024 Dec 29;15(1):62. https://doi.org/10.3390/diagnostics15010062 PMid:39795589 PMCid:PMC11719920
- Wang
YQ, Cao WJ, Gao YF, Ye J, Zou GZ. Serum interleukin-34 level can be an
indicator of liver fibrosis in patients with chronic hepatitis B virus
infection. World J Gastroenterol. 2018 March 28;24(12):1312-1320. https://doi.org/10.3748/wjg.v24.i12.1312 PMid:29599606 PMCid:PMC5871826
- Inci
E, Turkay R, Nalbant MO, Yenice MG, Tugcu V. The value of shear wave
elastography in the quantification of corpus cavernosum penis rigidity
and its alteration with age. Eur J Radiol. 2017 Apr;89:106-110. https://doi.org/10.1016/j.ejrad.2017.01.029 PMid:28267524
- Palabiyik
FB, Inci E, Turkay R, Bas D. Evaluation of Liver, Kidney, and Spleen
Elasticity in Healthy Newborns and Infants Using Shear Wave
Elastography. J Ultrasound Med. 2017 Oct;36(10):2039-2045. https://doi.org/10.1002/jum.14202 PMid:28417472
- Ferraioli
G, Tinelli C, Dal Bello B, Zicchetti M, Filice G et al. Liver Fibrosis
Study Group. Accuracy of real-time shear wave elastography for
assessing liver fibrosis in chronic hepatitis C: a pilot study.
Hepatology. 2012 Dec;56(6):2125-33. https://doi.org/10.1002/hep.25936 PMid:22767302
- Bercoff
J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft
tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq
Control. 2004 Apr;51(4):396-409. https://doi.org/10.1109/TUFFC.2004.1295425 PMid:15139541
- Bavu
E, Gennisson JL, Couade M, Bercoff J, Mallet V et al. Non-invasive in
vivo liver fibrosis evaluation using supersonic shear imaging: a
clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol.
2011 Sep;37(9):1361-73. https://doi.org/10.1016/j.ultrasmedbio.2011.05.016 PMid:21775051
- European Association for the Study of the Liver (2025). EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. EASL. https://easl.eu/publication/easl-clinical-practice-guidelines-on-the-management-of-hepatitis-b-virus-infection-2025
- Kavak
S, Kaya S, Senol A, Sogutcu N. Evaluation of liver fibrosis in chronic
hepatitis B patients with 2D shear wave elastography with propagation
map guidance: a single-centre study. BMC Med Imaging. 2022 Mar
18;22(1):50. https://doi.org/10.1186/s12880-022-00777-7 PMid:35303822 PMCid:PMC8932279
- Liu
K, Ding Y, Wang Y, Zhao Q, Yan L et al. Combination of IL-34 and AFP
improves the diagnostic value during the development of HBV related
hepatocellular carcinoma. Clin Exp Med. 2023 Jun;23(2):397-409. https://doi.org/10.1007/s10238-022-00810-7 PMid:35347503 PMCid:PMC10224837
- Shoji
H, Yoshio S, Mano Y, Kumagai E, Sugiyama M. Interleukin-34 as a
fibroblast-derived marker of liver fibrosis in patients with
non-alcoholic fatty liver disease. Sci Rep. 2016 July 1;6:28814. https://doi.org/10.1038/srep28814 PMid:27363523 PMCid:PMC4929441
- Preisser
L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED et al.
IL-34 and macrophage colony-stimulating factor are overexpressed in
hepatitis C virus fibrosis and induce profibrotic macrophages that
promote collagen synthesis by hepatic stellate cells. Hepatology. 2014
Dec;60(6):1879-90. https://doi.org/10.1002/hep.27328 PMid:25066464
- Castera
L. Non-invasive methods to assess liver disease in patients with
hepatitis B or C. Gastroenterology. 2012 May;142(6):1293-1302.e4. https://doi.org/10.1053/j.gastro.2012.02.017 PMid:22537436
- Cassinotto
C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J et al. Non-invasive
assessment of liver fibrosis with impulse elastography: Comparison of
supersonic shear imaging with ARFI and FibroScan®. J Hepatol.
2016;64(5):1047-1054. https://doi.org/10.1016/j.jhep.2016.01.003
- Myers
RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M et al.
Controlled Attenuation Parameter (CAP): a non-invasive method for the
detection of hepatic steatosis based on transient elastography. Liver
Int. 2012;32(6):902-910. https://doi.org/10.1111/j.1478-3231.2012.02781.x PMid:22435761
- Wei
H, Jiang HY, Li M, Zhang T, Song B. Two-dimensional shear wave
elastography for significant liver fibrosis in patients with chronic
hepatitis B: A systematic review and meta-analysis. Eur J Radiol. 2020
Mar;124:108839. https://doi.org/10.1016/j.ejrad.2020.108839 PMid:31981878
- Zhuang
Y, Ding H, Zhang Y, Sun H, Xu C et al. Two-dimensional Shear-Wave
Elastography Performance in the Non-invasive Evaluation of Liver
Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum
Fibrosis Indexes. Radiology. 2017 Jun;283(3):873-882. https://doi.org/10.1148/radiol.2016160131 PMid:27982760
- Kim
WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee
of the American Association for the Study of Liver Disease. Serum
activity of alanine aminotransferase (ALT) as an indicator of health
and disease. Hepatology. 2008 Apr;47(4):1363-70. https://doi.org/10.1002/hep.22109 PMid:18366115