In this study, we investigated the risk factors associated with an increased risk of multiple CMV reactivations or the development of CMV disease. We also evaluated the impact of these multiple CMV reactivations or disease on Overall Survival (OS), Progression-Free Survival (PFS), and Non-Relapse Mortality (NRM) in patients treated with LTV as prophylaxis against CMV reactivation after allo-HSCT. We retrospectively included 236 patients consecutively treated in our Center between January 2019 and May 2024. Conditioning intensity was determined according to the Transplant Conditioning Intensity (TCI) index.[12]
The median age at transplant was 55 years (range 17-72). All the patients had a positive CMV serology before allo-HSCT; 124 patients (52.5%) received allo-HSCT from a CMV-seropositive donor, while 111 (47%) received allo-HSCT from a CMV-seronegative donor; 1 donor's CMV serology was unknown. One hundred and twelve patients (47.5%) were affected by acute myeloid leukemia (AML), 32 (13.6%) by acute lymphoblastic leukemia (ALL), 25 (10.6%) by primary myelofibrosis, 22 (9.3%) by myelodysplastic syndrome (MDS) and 45 (19.1%) by other hematological diseases (such as chronic myeloid leukemia, CML, or chronic myelomonocytic leukemia, CMML). The Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) was 0 in 19 patients (38.1%), 1 or 2 in 68 (28.8%), and 3 or more in 78 (33.1%). Twenty-three patients (affected by myelofibrosis, MDS, CML, or CMML) (9.7%) underwent upfront allo-HSCT, without receiving any line of therapy before transplantation, while 129 (54.7%) received one line of therapy, and 84 (35.6%) received 2 or more lines of therapy before transplantation. Donors were an identical sibling donor in 23 cases (9.7%), a haploidentical donor in 35 (14.8%), a matched unrelated donor (MUD) in 115 (48.7%), a mismatched unrelated donor (MMUD) in 51 (21.6%), and a cord blood unit (CB) in 12 (5.1%). The stem cell source was bone marrow in 6 (2.5%), peripheral blood stem cells in 218 (92.4%), and CB in 12 patients (5.1%). Eighty-four patients (35.6%) received a myeloablative conditioning regimen (MAC), and 152 (64.4%) a reduced intensity one (RIC). T-cell-depletion for GvHD prophylaxis was performed in vivo with antithymocyte globulin (ATG) in 174 patients (74%), post-transplantation cyclophosphamide in 55 (23%), and alemtuzumab in 2 patients (1%). With a median follow-up of 2 years, CMV reactivation was observed in 62 patients (26.3%) with a median onset of 152.5 days (range 1-677) after transplantation. Notably, only 8 out of 236 patients (3.4%) had CMV reactivation during LTV prophylaxis (i.e., during the first 100 days after allo-HSCT), while in the remaining 54 cases, CMV reactivation occurred after the discontinuation of LTV. Twelve patients (5.1%) experienced ≥3 episodes of CMV reactivations, and in 9 patients (3.8%) a diagnosis of CMV disease was proven, including cases of colitis (N= 5) and pneumonia (N= 4), which resulted in one death; among these cases of CMV disease, only 2 cases of colitis occurred during prophylaxis with LTV. One hundred sixty-three patients (69.1%) developed aGvHD, and 101 (42.8%) cGvHD of any severity grade; overall, 128 patients (54.2%) received systemic therapy for acute or chronic GvHD (including corticosteroids, immunosuppressive drugs, or ruxolitinib).
Age ≥55 years was associated with a higher incidence of multiple CMV reactivations (p=0.048). Donor’s CMV-seronegativity was not associated with a higher incidence of either multiple CMV reactivations or CMV disease (p=0.43 and p=0.25, respectively). Additional factors, such as the HCT-CI score, the number of lines of therapy before transplantation, the HLA-relation between recipients and donors, the stem cell source, and the intensity of the conditioning regimen, did not impact the incidence of either multiple CMV reactivations or CMV disease.
While neither acute nor chronic GvHD significantly increased the probability of multiple CMV reactivations (OR 5.21 (0.99-96.16), p=0.117, and 2.82 (0.86-10.81), p=0.099, respectively), cGvHD was associated with a higher incidence of CMV disease (OR 4.95 (1.17-33.74), p=0.049), differently from aGvHD (p=0.22). The need for post-transplant steroid therapy did not affect the incidence of CMV disease (p=0.45), but it favored the development of multiple CMV reactivations (p=0.056). A CD4+ count ≤160/mmc and a CD3+ lymphocyte count ≤420/mmc at day 180 after allo-HSCT were associated with a higher incidence of multiple CMV reactivations (p=0.017 and p=0.004, respectively). Importantly, having more than 2 CMV reactivations was associated with a trend to poorer outcomes (1-year OS 84% vs 67%, p=0.046, and 1-year PFS 75% vs 67%, p=0.36) (Figure 1A-B). Furthermore, multiple CMV reactivations significantly increased the risk of 1-year NRM (25% vs 5% without multiple reactivations, p=0.001) (Figure 1C).
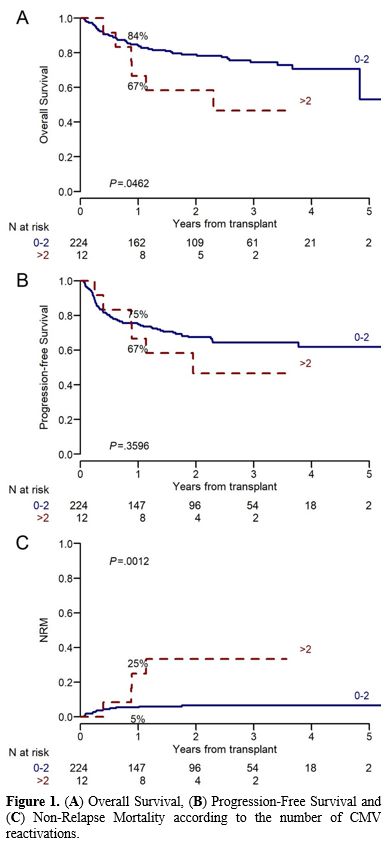 |
|
Our real-life data confirms the significant efficacy of LTV prophylaxis in reducing CMV reactivations and, more importantly, CMV diseases after allo-HSCT. This effect is associated with improved clinical outcomes and a reduction in NRM, without the emergence of relevant toxicities. Additional strengths include the relatively large sample size and the experience from a single center, which includes consecutive patients treated in a homogeneous manner. On the other hand, the most significant limitation of this study is its retrospective design.
In conclusion, this real-life experience confirms that despite the use of LTV prophylaxis, some patients still experience multiple CMV reactivations or develop CMV disease. This is particularly evident in patients with aGvHD or cGvHD, who are undergoing steroid therapy or have delayed T-cell recovery after transplantation. For these patients, continuing a close monitoring of the CMV viral load after 100 days post-allo-HSCT and considering an extension of LTV prophylaxis for up to six months after transplant could be highly beneficial.
References
- Schmidt-Hieber, M. et al. The prognostic impact of
the cytomegalovirus serostatus in patients with chronic hematological
malignancies after allogeneic hematopoietic stem cell transplantation:
a report from the Infectious Diseases Working Party of EBMT. Ann
Hematol 98, 1755-1763 (2019). https://doi.org/10.1007/s00277-019-03669-z PMid:30993417
- Ganepola,
S. et al. Patients at high risk for CMV infection and disease show
delayed CD8+ T-cell immune recovery after allogeneic stem cell
transplantation. Bone Marrow Transplant 39, 293-299 (2007). https://doi.org/10.1038/sj.bmt.1705585 PMid:17262060
- Stern,
L. et al. Human cytomegalovirus latency and reactivation in allogeneic
hematopoietic stem cell transplant recipients. Front Microbiol 10,
(2019). https://doi.org/10.3389/fmicb.2019.01186 PMid:31191499 PMCid:PMC6546901
- Ljungman, P. CMV infections after hematopoietic stem cell transplantation. Bone Marrow Transplant 42, (2008). https://doi.org/10.1038/bmt.2008.120 PMid:18724309
- Ljungman,
P., Hakki, M. & Boeckh, M. Cytomegalovirus in hematopoietic stem
cell transplant recipients. Infect Dis Clin North Am 24, 319-337
(2010). https://doi.org/10.1016/j.idc.2010.01.008 PMid:20466273
- Marty,
F. M. et al. Letermovir Prophylaxis for Cytomegalovirus in
Hematopoietic-Cell Transplantation. New England Journal of Medicine
377, 2433-2444 (2017). https://doi.org/10.1056/NEJMoa1706640 PMid:29211658
- Herrera,
F. et al. Letermovir primary cytomegalovirus prophylaxis in allogeneic
hematopoietic cell transplant recipients: could infection and disease
no longer be a significant problem? Letermovir and Cytomegalovirus
Prophylaxis in HSCT, Mediterranean Journal of Hematology and Infectious
Diseases, 16(1), p. e2024039 (2024). https://doi.org/10.4084/MJHID.2024.039 PMid:38882462 PMCid:PMC11178052
- Frietsch
J.J. et al. Resistance of cytomegalovirus towards letermovir due to
C325Y mutation in UL56 in a patient after hematopoietic stem cell
transplantation. Mediterr J Hematol Infect Dis 2019, 11(1): e2019001. https://doi.org/10.4084/mjhid.2019.001 PMid:30671207 PMCid:PMC6328044
- Gabanti,
E. et al. Human Cytomegalovirus-Specific T-Cell Reconstitution and
Late-Onset Cytomegalovirus Infection in Hematopoietic Stem Cell
Transplantation Recipients following Letermovir Prophylaxis. Transplant
Cell Ther 28, 211.e1-211.e9 (2022). https://doi.org/10.1016/j.jtct.2022.01.008 PMid:35042012
- Girmenia,
C. et al. The Changing Impact of Human Cytomegalovirus Serology and
Infection on Patient Outcome after Allogeneic Hematopoietic Stem Cell
Transplantation: An Italian Prospective Multicenter Survey in the Era
of Letermovir Prophylaxis. Open Forum Infect Dis 12, (2025). https://doi.org/10.1093/ofid/ofaf233 PMid:40322267 PMCid:PMC12048776
- Russo,
D. et al. Efficacy and safety of extended duration letermovir
prophylaxis in recipients of haematopoietic stem-cell transplantation
at risk of cytomegalovirus infection: a multicentre, randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Haematol 11,
e127-e135 (2024). https://doi.org/10.1016/S2352-3026(23)00344-7 PMid:38142695
- Spyridonidis,
A. et al. Redefining and measuring transplant conditioning intensity in
current era: a study in acute myeloid leukemia patients. Bone Marrow
Transplant 55, 1114-1125 (2020). https://doi.org/10.1038/s41409-020-0803-y PMid:31996792