Both primary plasma cell leukemia (pPCL) and secondary plasma cell leukemia (sPCL) are very uncommon subtypes of MM, with pPCL being more frequent than sPCL; however, the frequency of MM transformation into sPCL is increasing as patients survive longer. The molecular basis of sPCL remains poorly understood. sPCL is particularly aggressive and is associated with an extremely poor prognosis, constituting a major unmet medical need. High-quality data in PCL regarding presentation, treatment, and outcomes is limited. Herein, we review the current state of knowledge on PCL diagnostics, molecular biology, clinical characteristics, prognosis, and reported treatment outcomes, as well as the emergence of new therapeutic strategies. From 1960 to 2008, of newly diagnosed MM patients seen at the Mayo Clinic, 1.3% were diagnosed with PCL.[4] The crude incidence of primary plasma cell leukemia (pPCL) in Europe is 0.4 new cases per million.[4] Among 75399 patients with MM in the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2009, PCL accounted for approximately 0.6%.[5] Generally, pPCL accounts for about 60–70% of all PCL cases, while secondary plasma cell leukemia (sPCL) constitutes the remaining 30–40%.[4] These statistics were made when the definition of PCL was based on the presence of an absolute count of plasma cells ≥2 ×10^9/l and/or ≥20% circulating plasma cells (CPCs) in PB.[1,3] Today, according to the International Myeloma Working Group, the diagnosis of plasma cell leukemia is generally based on the percentage of CPCs in peripheral blood, which should be greater than 5%.[5,6] The count can be performed on a Giemsa-stained blood smear, but a cytofluorimetric count is preferred. However, it has been shown that patients with 2%-5% CPCs have similar outcomes to those with 5%-20% CPCs.[6,7] Therefore, 2% CTCs could serve as a biomarker of occult primary PCL and aid in assessing CPCs by flow cytometry during the diagnostic workup of MM.[8-10] Patients with CPCs in the range of 2% to 20% represent about 4% of the whole cohort and have shorter PFS than patients with CPCs
<2% (3.1 vs 15.6 months, respectively), as well as shorter OS (14.6 vs 33.6 months, respectively).[8] The proportion of sPCL among all MM is increasing, likely due to the new classification. Furthermore, some differences may exist among different ethnic groups. In Chinese papers, the incidence of patients with pCPCs >20% could be more than 5% of all MM, and those with CPCs >5% could be more than 10%.[9,10]
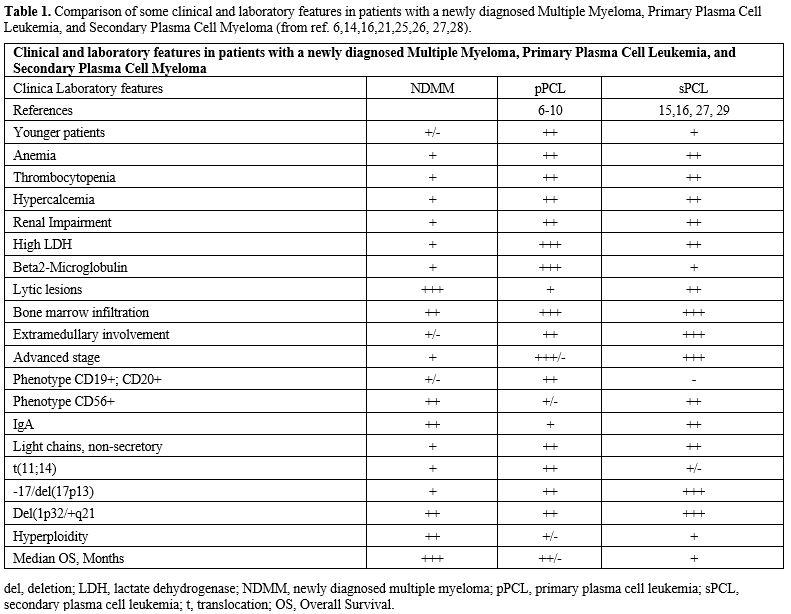
Patients in the 2-20% group had a higher frequency of ISS III stage, elevated LDH levels, and high risk cytogenetic abnormalities.[8-10] Finally, patients with 2-20% CTPCs have a prognosis comparable to that of patients with primary PCL.[8-11] In conclusion, some studies have demonstrated that a CTPC cut-off enables the identification of a subgroup of ultra-high-risk MM patients.[8-11] All these observations strongly support the inclusion of CTPC evaluation by flow cytometry as a standard part of the diagnostic workup of MM patients.[8-11] The clinical presentation of PCL is more aggressive than that observed in MM, including more severe cytopenia, hypercalcemia, and renal insufficiency. Higher tumor burden and PCL proliferation activity are reflected in higher levels of B2-microglobulin and lactate dehydrogenase (LDH) (Table 1). Extramedullary involvement (lymph nodes, liver, spleen, pleura, and central nervous system) at diagnosis is more common in pPCL and sPCL than in MM. Still, osteolytic lesions are more frequent in sPCL and MM than in pPCL.[13-16] A further sign of a bad prognosis is the presence of extraosseous localization (Table 1).[17-18]
 |
|
Immunophenotype
Various studies have analyzed the immunophenotype of PCL. The two common MM markers, CD38 and CD138 antigens, are similarly expressed in MM and PCL. Immunophenotypic expressions were similar for CD38, CD138, CD2, CD3, CD16, CD10, CD13, and CD15, but PCL differed from MM in the expression of CD56, CD9 HLA-DR, CD117, and CD20 antigens.[18] However, PCL displays a more immature phenotype than MM, expressing more frequently CD20, CD23, CD28, CD44, and CD45, and less frequently CD9, CD56, CD71, CD117, and HLA- DR antigens.[18-22] Furthermore, there are differences in the immunophenotypes of pPCL and sPCL. FC is an excellent method for identifying circulating PCs[7,19] as a significantly higher number was identified by FC than by morphology (267% vs. 135%, P = 002). None of the secondary PCL cases expressed CD19 or CD20. A low level of expression, with similar positivity for CD27, CD28, CD81, and CD117, was observed in both PCL groups. A decrease in CD44 expression was detected only in secondary PCL.[15,16]Numerous features distinguish PCL from standard MM, including molecular features (at the genomic and gene expression levels), phenotypic and bone marrow microenvironment features, different frequencies of cytogenetic abnormalities, a low proportion of hyperdiploidy, impaired renal function, high tumor burden, and high LDH levels (Table 1).
Cytogenetic and Molecular Features
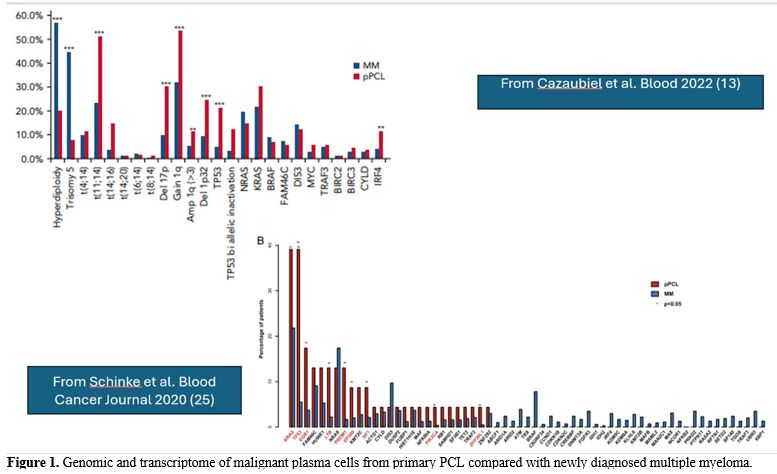
Few studies have reported an extensive molecular characterization of relatively large cohorts of pPCL (Table 1). A fundamental study by Cazuabiel et al.[23] reported targeted DNA and RNA sequencing results from 96 pPCL samples and compared them with those observed in a large cohort of MM patients. The main findings of this genomic analysis were: (i) a lower frequency of hyperploidy compared to MM (20% vs 57%, respectively); (ii) high prevalence of some IgH translocations with higher frequency in pPCL than in MM, such as t(11;14) (51% vs 23%, respectively), t(14;16) (14% vs 3%), while other IgH translocations were observed at comparable frequencies, such as t(4;14) (11% vs 10%, respectively); (iii) several adverse cytogenetic abnormalities are more frequent in pPCL than in MM, such as del(17p) (30% vs 9.5%, respectively), 1q gain (56% vs 32%) and del (1p32) (24% vs 9%); (iv) some gene mutations, such as TP53 (21% vs 5%) and IRF4 (11% vs 4%) were more frequent in pPCL than in MM; (v) biallelic TP53 inactivation was much more frequent in pPCL than in MM (17% vs 3%, respectively).[23]At the gene expression level, genes involved in MYC targets and G2M checkpoint are significantly more expressed in pPCL than in MM.[24] A whole-exome sequencing analysis conducted by Schinke et al.[25] in a small cohort of PCL patients confirmed these findings, revealing the accumulation of complex structural changes and adverse genetic events in these tumors, which could account for the poor outcomes in this patient group. The number of mutations is higher in pPCL than in MM, seemingly due to a greater prevalence of the APOBEC mutational signature induced by MAF/MAFB translocations.[25] This study confirmed a markedly reduced frequency of hyperploidy and a strongly increased frequency of gains of chromosome 1 and of t(14;16) or t(14;20) in pPCL compared to MM.[25] Several driver genes, such as KRAS, TP53, EGR1, LTB, PRDM1, EP300, NF1, PIK3CA, and ZFP361, are significantly more frequently mutated in pPCL than in MM.[25] In a more recent study, Cazaubiel et al. evaluated the molecular heterogeneity of PCLs and defined two subgroups with distinct genomic and transcriptomic features, based on the presence or absence of the t(11;14) translocation.[26] These two subgroups differ in the frequency of chromosome 1 abnormalities, such as amp1q, gain 1q, and del1p32, which is much higher in the subgroup without t(11;14) than in the subgroup with t(11;14)[26] (Table 1 and Figure 1). Importantly, the cases with two or more lesions, including del 17p, gain/amp 1q, and/or del 1p, are much more frequent among PCLs without t(11;14) compared with those with this translocation.[25] In line with these findings, PCLs with t(11;14) have a better prognosis than those without t(11;14).[26] In addition, van de Donk et al. reported that the cytogenetic profile differed between patients aged ≤65 years and those aged >66 years enrolled in the pPCL EMN12/HOVON-129 study.[27] The authors reported that the frequency of t(11;14) was lower, whereas the frequencies of t(14;16) and del(17p) were higher in patients with pPCL aged ≤65 years.
 |
|
Bruinink and coworkers identified a peculiar transcriptomic profile associated with pPCL and PCL-like MM characterized by higher levels of CTPCs.[8] This transcriptomic classifier was identified from the analysis of genes differentially expressed in pPCL and MM samples; after selecting the 54 most differentially expressed genes, it included genes involved in cell adhesion, tumor suppression, proliferation, RNA splicing, cell migration, and DNA damage control.[8] This transcriptomic classifier identified 94% of pPCL and a subgroup of newly identified MMs, defined as PCL-like; these PCL-like tumors were identified in 15% of MMs, 2% of MGUS, and 8% of SMM.[27] At the cytogenetic level, PCL-like MMs are more similar to PCLs than to non-PCL-like MMs (i.e., iMMs); several remarkable cytogenetic differences have been observed between PCL-like MMs and iMMs. 53% of PCL-like MMs have CTPC values of 2%. The analysis of a large cohort of 2,139 MM patients showed that PCL-like MMs have a significantly shorter PFS and OS compared to iMMs.
Secondary PCL (sPCL) represents the leukemic transformation of a preexisting MM. Some remarkable differences distinguish sPCL from pPCL. Thus, an initial study by Gonzalez-Paz et al. showed that sPCL harbors t(11;14) much less frequently than pPCL; in contrast, t(4;14) and t(16;14) are more frequent in sPCL than in pPCL; TP53 mutations are more frequent in pPCL than in sPCL.[14] Papadhimitriou et al.[28] reported the molecular characterization of 25 pPCL and 19 sPCL, showing that: (i) sPCL had more cytogenetic abnormalities than pPCL; del(13q) was more frequent in sPCL (94.7%) than in pPCL (59.1%); del(17p) was markedly more frequent in sPCL (68%) than in pPCL (16%); t(11;14) was present only in pPCL (52%) and absent in sPCL; t(4;14) and chromosome 1 alterations displayed a trend toward an higher frequency in sPCL; MYC translocations were more frequent in pPCL than in sPCL.[28,29]
Skerget et al.[30] examined the gene expression profiles of pPCL and sPCL and found that 26% of pPCL and 83% of sPCL exhibit a proliferation signature associated with poor outcomes. Interestingly, some patients who transitioned to a proliferation profile acquired genetic alterations, such as biallelic deletions of CDKN2C or RB1, which predisposed them to a proliferation phenotype.[30] Patients with a PR profile have a significantly shorter OS than those without a PR phenotype.[30]
Lee et al. have explored the mutational profile of malignant plasma cells in BM and in PB in a few patients with pPCL and sPCL.[31] This analysis revealed that while the majority of variants were detected in both BM and PB plasma cells, some variants were selectively found in either BM or PB plasma cells.[30] In pPCL samples, MED12 and VPS13B variants were observed only in PB, while ZMY/M3 variants were observed only in BM.[32] In pPCL samples, shared variants were expressed at higher allele frequencies in PB than in BM.[32,33]
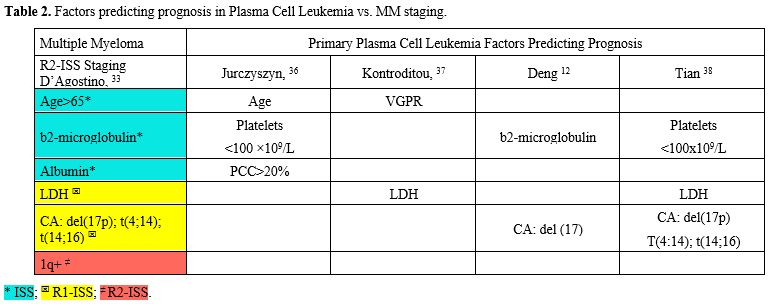
Prognosis
As in classical multiple myeloma, pPCL prognosis is influenced by host characteristics, tumor burden (stage), biology (including cytogenetic abnormalities), and response to therapy. The existing risk models for multiple myeloma (MM) are suboptimal for stratifying patients with primary plasma cell leukemia (pPCL), considering that the percentage of circulating plasmablasts is not included among the parameters of all types of R-ISS;[34] it is currently considered a significant sign that worsens the prognosis.[11,24,34] So, a proportion of patients with PCL remain in stages I and II.[12,15,33] In general, the prognosis of pPCL is worse than that of MM, but better than that of sPCL.[14-16,28,30,32,33] However, not all pPCL have the same prognosis; therefore, over the last few years, several proposals have been made for specific indicators in pPCL (Table 2).[8-12,34]An European American multicenter retrospective study analyzed clinical characteristics and outcomes in 117 patients with primary plasma cell leukemia (pPCL) treated at the participating institutions between January 2006 and [missing information] December 2016. The median age at pPCL diagnosis was 61 years. Ninety-eight patients were treated with novel agents, with an overall response rate of 78%.
Multivariate analyses identified age ≥ 60 years, platelet count ≤100 × 109/L, and peripheral blood plasma cell count ≥20 × 109/L as independent predictors of worse survival. The median OS in patients with 0, 1, or 2–3 of these risk factors was 46, 27, and 12 months, respectively (P < 0_001). These findings support the use of novel agents and ASCT as frontline treatment in patients with pPCL.[36]
In the same year, 2018, the Greek Myeloma Study
Group retrospectively reviewed the medical records of 50 consecutive pPCL patients registered in the Greek Myeloma Study Group (GMSG) database between January 2000 and January 2016, among 2711 patients with MM. These patients were all treated with novel drugs, including bortezomib.[37] In the Cox regression analysis, they found that, in univariate analysis, Bortezomib-based therapy + ASCT predicted OS. In multivariate analysis, achieving very good partial remission (vgPR) and LDH ≥ 300 U/L were significant predictors of OS.
Similarly, a multicenter retrospective study was conducted across 16 hospitals in China.[12] A total of 102 pPCL patients were included in this study. The 12-month, 24-month, and 36-month OS rates for pPCL patients were 75.4%, 58.3%, and 47.6%, respectively. An overall survival prognostic nomogram for pPCL patients was developed by integrating independent prognostic factors. A nomogram incorporating age, b2MG grade, and del17p was developed and validated to accurately and consistently predict the prognosis of pPCL patients.[12]
A more comprehensive study[38] on the stratification of patients with primary plasma cell leukemia (pPCL) was recently conducted in China by Tian et al. They aimed to develop a staging system for pPCL, defined as ≥ 5% circulating plasma cells (CPC) according to the new diagnostic criteria, using one of the largest pPCL patient series, comprising 340 patients (the training cohort) from 25 centers across China. The prognostic impact of baseline features and cytogenetic abnormalities was assessed. Univariate and multivariate analyses identified variables predicting overall survival (OS) to create a staging system. Its performance was then validated in an independent cohort (n = 80). Genome-wide DNA and RNA sequencing explored the molecular basis for clinical differences between stages. Del(17p), t(4;14), and t(14;16), but not 1q+, were confirmed as high-risk cytogenetic abnormalities (HRCAs) of pPCL. Elevated LDH and thrombocytopenia also strongly affected OS. These findings contributed to a simple algorithm that stratifies pPCL patients into stages I, II, and III, with median OS of 54.1, 24.0, and 5.4 months, respectively (II vs. I: HR, 1.986; 95% CI, 1.034–3.814; P = 0.0394).
The model’s accuracy (c-index 0.711) exceeded that of other models. Furthermore, patients in different stages exhibited markedly distinct genomic and transcriptomic abnormalities.[38]
The articles cited so far do not take into account the location where patients were treated. This is a crucial factor, not only in distinguishing between low-income and high-income countries but also in considering the hospitals where patients are treated. In a recent article,[38] Saba and Colleagues distinguished between 30-day mortality and late mortality. They stressed in their study that the 30-day mortality was statistically influenced by physiological and social determinants, including male gender, increased comorbidity burden (CCI), and type of insurance.[39]
In conclusion, the parameters proposed for predicting the outcome of single cases of pPCL are not significantly different from those in the R2-ISS, but they involve fewer predictors (Table 2).
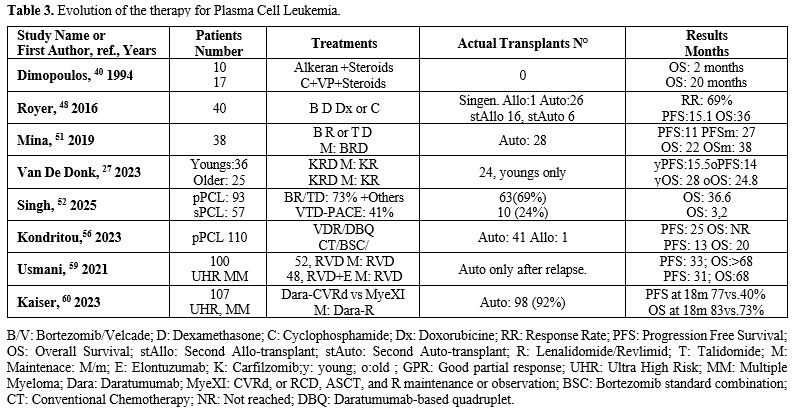
Therapy
Standard therapy for myeloma has been shown for many years to be ineffective in pPCL. The median life of patients treated with melphalan, prednisone, or VAD was a few months.[39] However, in the same paper, patients treated with a high-risk lymphoma-like cycle had a median survival of 22 months (Table 3). The efficacy of hematopoietic stem cell transplantation has been well documented, even before the introduction of new drugs for MM.[40-42] We reported many years ago a patient with primary plasma cell leukemia, resistant to VAD, who went into remission with Cyclophosphamide and Vepesid and then underwent transplantation with CD34-positive selected cells. She is in complete remission and well at present, thirty years later.[41] The introduction of bortezomib was fundamental in myeloma therapy and similarly was in pPCL.[42-46] The addition of Bortezomib to Cyclophosphamide and dexamethasone improved overall survival in pPCL, particularly when combined with autologous hematopoietic stem cell transplant.[43,44] The addition of bortezomib was considered real progress.[43-48] Royer and Coll. treated 40 patients with Bortezomib, Cyclophosphamide, Doxorubicin, and Dexamethasone, observing a median OS and PFS of 36.3 and 15.1 months, respectively. The 12-month PFS was 58%, and the 12-month OS was 75%. The ORR to induction was 69%, which included VGPR or better in 36% (n = 14), and the best response achieved during the entire program was VGPR or better in 59% (n = 23).[48] Similarly, Pagano and colleagues[49] achieved a 55% CR by combining bortezomib with high-dose cyclophosphamide and double autologous stem cell transplantation (aHSCT). The median OS of patients who achieved CR was not reached, and their PFS was 50 months. Following the evolution of treatment for patients with a new diagnosis of newly diagnosed multiple myeloma (NDMM) in transplant-eligible (TE) patients,[50] the pPCL treatment has also changed.However, the prognosis, particularly of sPCL patients, remained poor. Tessier et al., in a retrospective analysis involving 33 pPCL and 66 sPCL treated from 2005 to 2020, observed a mOS for pPCL and sPCL of 18.3 months and 1.3 months, respectively; in pPCL patients, autologous HSCT improved OS compared to chemotherapy alone.[15] The impact of HSCT on patients with sPCL cannot be assessed because these patients were rarely transplanted.[12] These investigators did not observe improvement in OS over time (2005–2012 vs. 2013–2020, p=0.629 for pPCL and p=0.329 for sPCL); after 2012, bortezomib was introduced in the therapy of all patients with pPCL. Mina and coworkers[51] reported treatment response in 38 pPCL patients undergoing a bortezomib-based induction regimen. In 92% of these patients, the response was associated with an immunomodulatory drug, and 74% underwent aHSCT.[51] The ORR was 87%, with 45% of CR; the mPFS was 20 months, and the mOS was 33 months; PFS was prolonged in patients undergoing aHSCT in comparison with those who did not undergo aHSCT (25 vs 6 months); patients who received maintenance therapy after aHSCT displayed prolonged mPFS 27 vs 11 months) compared to those who did not receive maintenance therapy.[50] The maintenance therapy included Revlimid, Bortezomib (Velcade), and Dexamethasone (lenalidomide, bortezomib, and dexamethasone) more frequently or one or 2 of these drugs.[50]
A large retrospective analysis of 153 PCL patients, using the new definition of ≥5% circulating plasma cells, was conducted across seven academic centers in the United States, examining clinical features and treatment outcomes.[52] This is one of the largest multicenter studies conducted in the US on this rare disease, involving 93 patients with primary PCL and 57 with secondary PCL. Additionally, associations between patient characteristics and mortality were investigated using Cox proportional hazards regression models. This paper confirms well-known data in numerous cases, such as the association of secondary PCL with a worse prognosis, showing an overall survival of 3.2 months compared to 36.6 months for primary PCL (P<0.001). Receipt of a transplant was associated with a survival advantage in both primary PCL (Hazard Ratio [HR] = 0.16, P < 0.001) and secondary PCL (HR = 0.20, P = 0.001). No significant difference in outcomes was observed between proteasome inhibitor-based triplet regimens and the VTD-PACE-like regimen. The presence of extramedullary disease and high-risk cytogenetics was not associated with survival in the primary PCL group (Table 3).[52]
Like other forms of myeloma, the three-drug induction regimen combining a proteasome inhibitor, such as bortezomib, an immunomodulatory drug, such as lenalidomide, and dexamethasone (RVd) was believed to be the most promising frontline induction therapy at the time; however, the PCL shows a reduced response rate.[51,52] The antibodies to CD38, used successfully in other forms of myeloma, have been shown to be effective in relapsed or resistant PCLs.[53-54] However, in patients with a new diagnosis, there are no randomized studies demonstrating a statistically significant advantage in outcomes with a Triplet such as RVD, even though the study by Li et al. showed that daratumumab in the therapy significantly prolongs OS.[55]
A more modern proteasome inhibitor, carfilzomib, has been used as a substitute for Bortezomib (Velcade) in the therapy of pPCL.The results of the non- randomized, phase II study EMN12/HOVON-129 were released in 2023.[56] This study involved 61 newly diagnosed pPCL (NDPCL) patients treated with carfilzomib, lenalidomide, and dexamethasone (KRd) induction, followed by double aHSCT, four cycles of KRd consolidation, and then maintenance with carfilzomib and lenalidomide until progression. With a median follow-up of 43.5 months, the median progression-free survival (mPFS) was 15.5 months for younger patients and 13.8 months for older patients. Although these results show an improvement in PFS compared with previous studies, they remain clearly inferior to MM in pPCL.[27]
Katodritou et al. (2023) retrospectively analyzed 110 pPCL patients undergoing treatment with either the bortezomib-lenalidomide triplet (VRd) or the daratumumab-based quadruplet (DBQ), compared with previous therapies such as conventional chemotherapy (CT) or bortezomib standard combinations (BSC).[56] Treatment with VRd/DBQ correlated with a higher complete response rate (41% vs 17%), longer PFS (25 months vs 13 months), and OS (not reached vs 20 months) (Table 3).[56]
Recently, Shalabi and others[57] identified 30 patients with pPCL and 29 patients with sPCL.
Of the entire cohort, 51.9% received an induction regimen with novel agents, excluding chemotherapy. Of the evaluable patients with pPCL and sPCL, 82.1% (23/28) and 64.7% (11/17), respectively, achieved a partial response or better. Median PFS was significantly worse in patients with sPCL than pPCL (2.2 vs. 38.3 months; HR 0.16; 95% CI (0.07-0.35), P ≤.001). Median OS was also worse in patients with sPCL than in those with pPCL (3.1 months vs. NR [not reached]; HR 0.09; 95% CI 0.04-0.23; P ≤ .001). The median post-SCT survival for patients with pPCL was NR compared with 6.7 months for patients with sPCL (HR 0.17; 95% CI (0.03-0.83), P = .03).
The median time to transform into sPCL in patients who received Daratumumab (Dara)- containing regimens for multiple myeloma was 46.8 months, compared with 12.3 months in patients who didn’t (P = .007). Dara's refractory status was associated with worse OS (HR 5.63; 95% CI (2.75-11.51), P ≤0.001).
Therapy of High-Risk myeloma, including pPCL
Considering that there are many MM with circulant neoplastic plasma cells, which are a significant risk factor, and have a prognosis not so different from plasma cell leukemia,[8-11] there is a tendency not to consider the PCL a distinct entity, but to classify it in a group of ultra-high-risk (UHR) myeloma together with the extramedullary myelomas and multi-hit myeloma (the co-occurrence of two or more high-risk cytogenetic abnormalities), extramedullary disease, plasma cell leukemia, and a high-risk gene expression profiling signature have emerged as defining features of ultrahigh- risk multiple myeloma (uHRMM).[58] Consequently, in some recent trials, the pPCLs were classified together with high-risk molecular features MM. The SWOG 1211 trial randomly assigned MM patients with high-risk molecular features (t(14;16), t(14;20), del(17p), ampl(1q21) or pPCL to treatment with VRD (bortezomib/Velcade, lenalidomide/Revlimid, and dexamethasone) alone or in combination with elotuzumab (RVD-E); however, RVD-E treatment failed to improve PFS over RVD (median PFS 31.5 vs 36.4 months) (Table 3 and Figure 2).[59]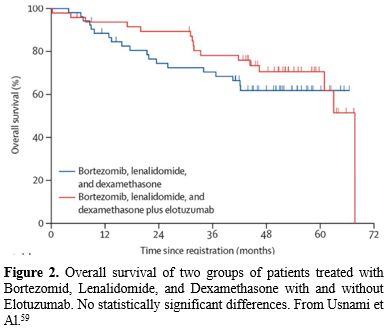 |
|
However, the prognosis, particularly of sPCL patients, remained poor. Tessier et al., in a retrospective analysis invol
The multicenter OPTIMUM phase II trial 876) investigated treatment based on daratumumab, low- dose cyclophosphamide, lenalidomide, bortezomib and dexamethasone (Dara-CVRD) before and after ASCT in MM patients with ultra-high-risk disease, including pPCL patients: This treatment was compared to the results obtained in comparable MM patients treated in the context of MyeX1 trial (patients treated with carfilzomib, lenalidomide, dexamethasone and cyclophosphamide or lenalidomide, daratumumab and dexamethasone). At a 30-month follow-up, PFS was 77% with OPTIMUM versus 39.8% with MyeXI, and OS was 83.5% with OPTIMUM versus 73.5% with MyeXI.[60]
The GMMG-CONCEPT trial (2024) investigated isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRD) in transplant-eligible (TE) and transplant-noneligible (TNE) patients with MM with high-risk molecular features or with pPCL.[61] The patients achieved a MRD negativity after consolidation therapy of 67.7% among the TE patients and of 54.2% in TNE patients; MRD negativity was sustained for 1 year in 62.6% of patients; with a median follow-up of 44 months (TE patients) or of 33 months (TNE patients), median PFS was not reached in either arm.[61]
Transplant Evaluation
Hematopoietic stem cell transplantation after an eradicating regimen constitutes a fundamental step in the PCL upfront treatment in fit patients. The increase of OS and PFS in transplanted patients compared to non-transplant patients is well- documented and widely accepted.[6,49,51,56]However, the modalities of transplantation are various and include Autologous Stem cell transplantation, double autologous aHSCT, Allogeneic Stem Cell Transplantation, aHSCT followed by alloHSCT, allo-HSCT followed by aHSCT.
Due to the rarity of PCL, only collaborative studies have sufficient numbers of patients to achieve statistical significance. Dhakal et al.[62] report patients in the CIBMTR (Center for International Blood & Marrow Transplant Research) Database, which registers patients from 450 centers, mostly in the USA. They report the outcomes of 348 patients with pPCL who received autologous (auto-) HCT (n = 277) or allogeneic (allo-) HCT (n = 71) between 2008 and 2015. Karnofsky performance status (KPS) > 90 and ≥ very good partial response (VGPR) predicted superior OS in multivariate analysis for auto-HCT. For allo-HCT, the 4-year outcomes were: NRM 12% (5–21%), REL 69% (56–81%), PFS 19% (10–31%), and OS 31% (19–44%).
Compared with prior CIBMTR pPCL patients (1995–2006), the current cohort had inferior survival (3-year OS: 39% vs. 38% in allo-HCT and 62% vs. 35% in auto- HCT). However, HCT utilization increased from 12% (7–21%) in 1995 to 46% (34–64%) in 2009, as reported in SEER data (available through 2009).[62]
Lawless et al. (2023)[63] made an updated, retrospective analysis of the European Blood and Marrow Transplantation Group (EBMT) based on 751 pPCL patients transplanted between 1998 and 2014, comparing four different modalities of transplant strategies, including single auto-SCT and single allo- SCT; with a median follow-up of 4 years, the mPFS and mOS of all these patients were 14 and 33 months, respectively.[63]
Initial comparisons were made between patients undergoing allo-first (n=70) versus auto-first (n=681), regardless of a subsequent second transplant. The allo- first group had a lower relapse rate (45.9%, 95% confidence interval [95% CI]: 33.2-58.6 vs. 68.4%, 64.4-72.4) but higher non-relapse mortality (27%, 95% CI: 15.9-38.1 vs. 7.3%, 5.2-9.4) at 36 months. Patients who underwent allo-first had a remarkably higher risk in the first 100 days for both overall survival and progression- free survival. Patients undergoing auto-allo (n=122) had no increased short-term risk and a significant benefit in progression-free survival after 100 days compared with those undergoing single auto (hazard ratio [HR]=0.69, 95% CI: 0.52-0.92; P=0.012). Auto-auto (n=117) was an effective option for patients who achieved complete remission prior to their first transplant, whereas in patients who did not, our modeling predicted that auto- allo was superior. This study confirms a significant mortality risk within the first 100 days for allo-first and suggests that tandem transplant strategies are superior. Disease status at the time of transplant influences outcome. This knowledge helps guide clinical decisions on transplant strategy.
Magaeki e al., for the group Stem Cell Transplantation for Primary Plasma Cell Leukemia in Japan, analyzed patients with pPCL who underwent HCT between 2006 and 2022 in Japan using Japanese registry data. Overall, 182 patients (117 and 65 who underwent auto-HCT and allo-HCT, respectively) were included. Patients in the allo-HCT group were younger, had more advanced disease, and underwent tandem HCT more frequently than did those in the auto-HCT group.
There was no statistically significant difference in overall survival (OS) between the groups (P=0.46), with a median OS of 3.2 years (95% CI 2.1–4.3 years) in the auto-HCT group and 1.4 years (95% CI 0.8–3.9 years) in the allo-HCT group. Among the four transplantation strategies (single auto-HCT, single allo-HCT, tandem autologous-autologous HCT, and tandem autologous- allogeneic HCT), the single allo-HCT group had the poorest OS due to early mortality. Tandem autologous- allogeneic HCT appeared to provide better long-term outcomes.
A matched-pair analysis using propensity scores revealed no significant differences in OS between the auto-HCT and allo-HCT groups. However, the allo-HCT group appeared to have better long-term survival than the auto-HCT group. Allogeneic transplantation, including tandem autologous-allogeneic-HCT, may offer long- term survival benefits with appropriate patient selection. Further studies are warranted to optimize the transplantation strategies and pre- and post- transplantation treatments for pPCL.
In conclusion, both the EBMT study and the Japanese study suggest better outcomes for patients treated with tandem autologous-allogeneic therapy, particularly those transplanted outside complete remission. However, all three trials highlight the poor results with respect to the other NDMM.
The higher relapse rates warrant the development of novel agents and clinical trials to improve transplant- related outcomes in this challenging subgroup.
Special Therapies and Resistant and Relapse Treatments
A triplet regimen with or without anti- CD38 immunoglobulins is usually needed at relapse, with the choice of regimen varying with each successive relapse. Chimeric antigen receptor T (CAR-T) cell therapy and bispecific antibodies are additional options.[65] However, primary plasma cell leukemias displaying t(11;14) have specific genomic, transcriptional, and clinical features.[13,66,67] This sub- entity showed significantly fewer adverse cytogenetic abnormalities, resulting in better overall survival than pPCL without t(11;14) (39.2 months vs 17.9 months, P = .002). Finally, pPCL with t(11;14) displayed a specific transcriptome, including differential expression of BCL2 family members. Given its high prevalence - more than 25% - in plasma cell leukemia and the limited treatment options available in this disorder, Venetoclax, a BCL-2 inhibitor, also shows promise in pPCL with t(11;14).[66,67] BCL-2 is an anti-apoptotic protein that promotes cancer cell survival, and myeloma cells with t(11;14) are particularly dependent on BCL-2. Venetoclax blocks BCL-2, activates the apoptotic pathway, and selectively eliminates BCL-2-dependent tumor cells. As a result, Venetoclax is especially effective in t(11;14)-positive multiple myeloma, often inducing deep and durable responses.[66,67] Venetoclax, either as monotherapy or in combination with daratumumab, bortezomib, and other agents, may be a viable treatment option for this subset of pPCL patients in relapse or resistant after the standard therapy. Several single cases have been reported.[68-78] Also, the sPCL with t(11;14) has shown a good response to Venetoclax.[77,88] In primary plasma cell leukemia with t(11;14) or BCL2 expression, Venetoclax has been utilized with success also upfront.[66]CAR T Cell and Bispecific Antibodies in relapse/Resistant Myeloma. The present guidelines do not cover CAR T cells or bispecific antibodies for frontline therapy in new diagnosis pPCL. The use of CAR-T cells in PCL patients was limited to those with resistant or relapsing diseases.[79] In this context, a recent study reported the results on the treatment of 11 PCL patients (4 pPCL and 7 sPCL) with Ide-Cel: all pPCL and 4/7 sPCL patients responded to treatment; in all patients, MPFS was 3.7 months and mOS was 6.7 months; patients with sPCL had shorter mOS than those with pPCL (4.6 vs 8.1 months).[80] In another study presented at the 2023 EHA congress,[81] 8 patients with sPCL were enrolled at a Chinese study center from 2020.12 to 2022.11. All patients were in the high-Risk group, with Double Hit Myeloma 8/8 (100%) patients were all resistant to lenalidomide, 4/8 (50%) were resistant to pomalidomide, 8/8 (100%) were all resistant to bortezomib, 2/8 (25%) were resistant to carfilzomib, and 8/8 (100%) were all resistant to daratumumab; only 3/8 (37.5%) patients had SCT history. The median and range of peak BCMA-CART amplification were 16 (11- 28) days; the best outcome after BCMA-CART was PR in 6 patients, with ORR at 1 and 2 months after CART of 6/8 (75%), 4 PR, 2 VGPR. Three of the six patients who achieved remission underwent Allo-SCT for consolidation therapy three months after CART, two patients are still alive with sCR, and one patient relapsed and died one month after consolidation therapy with Allo-SCT; the other three patients did not undergo Allo-SCT and consolidation therapy with CART for financial and medical reasons, and one relapsed and died 6 months after CAR T, one relapsed and died 1 year and 3 months after CAR T, and one is still alive. One case died after one year and 3 months after CAR T relapse, and one case is still in the VGPR follow-up phase. The median follow- up time was 186.5 days, and the 6-month PFS and OS rates were 62.5% and 60%, respectively. BCMA-CAR T treated CRS Grade 1 ratio was 4/8(50%), Grade 2 was 2/8(25%), Grade 4 was 2/8(25%); all 8 patients had no ICANS; anemia Grade 3 was 8/8(100%), neutropenia Grade2 was 1/8(12.5%), Grade 3 was7/8(87.5%), thrombocytopenia Grade 3 was 1/8(12.5), Grade 4 was 7/8(87.5%); nausea/vomiting Grade 1 was 4/8(50%) and Grade 3 was 4/8 (50%); two patients died within one month of returning to CART due to severe pneumonia and gastrointestinal hemorrhage; two patients who died early had up to 70% abnormal peripheral blood plasma cells; and one patient developed pulmonary Aspergillus infection three months after returning to CAR T.
Other single cases treated with momentary success have been reported.[82-86]
These results showed that treatment of PCL patients with CAR-T cells targeting CD38 is feasible, but outcomes remain poor. New targets on the malignant cells could permit a better response.[87]
Bispecific antibodies have shown good activity in relapsed and resistant myeloma patients;[88] however, to my knowledge, only one case of PCL, treated with bispecific antibodies, has been reported.[89] This patient experienced a very quick achievement of response, after only 1 cycle and allowed the patient to achieve an improved quality of life. However, the duration of response was short, 5 months on Elranatamab and 2 months on Talquetamab.
Conclusions
The independence of plasma cells from the bone marrow microenvironment marks a significant evolutionary step in disease biology, and it defines the disease categories of PCL and EMD. Recent studies have greatly contributed to identifying unique molecular abnormalities seen in EMM and PCL compared to other forms of MM. However, these studies have not identified specific genes or altered pathways that can be targeted in these tumors. The presence of 2%-5% CTCs mimics the outcomes of pPCL, regardless of standard risk factors. Both PCL and EMD are classified as ultrahigh-risk (uHR)MM, which leads to death within 24-36 months, and high-risk (HR)MM, which leads to death within 36-60 months. Advances in therapy have led to unprecedented survival rates in multiple myeloma. However, novel therapies have disproportionately benefited standard-risk patients, and an underserved high-risk population continues to experience early progression and mortality. Some studies using new agents have shown promising activity against EMM or PCL, but these findings need to be confirmed in future clinical trials focused on these tumors. Finally, it is essential to emphasize that research on EMM and PCL has underscored the importance of including, in the diagnostic evaluation of each newly diagnosed MM patient, an assessment of peripheral blood for the presence of malignant plasma cells.References
- Kyle RA, Maldonado JE, Bayrd ED. Plasma cell
leukemia. Report on 17 cases. Arch Intern Med. 1974 May;133(5):813-8.
doi: 10.1001/archinte.133.5.813. https://doi.org/10.1001/archinte.133.5.813 PMid:4821776
- de
Larrea CF, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, et al.
Plasma cell leukemia: consensus statement on diagnostic requirements,
response criteria and treatment recommendations by the International
Myeloma Working Group. Leukemia. 2013;27:780-9 https://doi.org/10.1038/leu.2012.336 PMid:23288300 PMCid:PMC4112539
- Gertz MA, Buadi FK. Plasma cell leukemia. Haematologica. 2010; 95:705-707. doi:10.3324/haematol.2009.021618 https://doi.org/10.3324/haematol.2009.021618 PMid:20442443 PMCid:PMC2864374
- Sant
M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies
in Europe by morphologic subtype: results of the HAEMACARE project.
Blood. 2010;116:3724-3734. doi:10.1182/blood-2010-0528263 https://doi.org/10.1182/blood-2010-05-282632 PMid:20664057
- de
Larrea CF, Kyle R, Rosiñol L, et al. Primary plasma cell leukemia:
consensus definition by the International Myeloma Working Group
according to peripheral blood plasma cell percentage. Blood Cancer J.
2021;11:192. doi:10.1038/s41408-021-00587-0. https://doi.org/10.1038/s41408-021-00587-0 PMid:34857730 PMCid:PMC8640034
- Musto
P, Engelhardt M, van de Donk NWCJ, Gay F, Terpos E, Einsele H,
Fernández de Larrea C, Sgherza N, Bolli N, Katodritou E, Gentile M,
Royer B, Derudas D, Jelinek T, Zamagni E, Rosiñol L, Paiva B, Caers J,
Kaiser M, Beksac M, Hájek R, Spencer A, Ludwig H, Cavo M, Bladé J,
Moreau P, Mateos MV, San-Miguel JF, Dimopoulos MA, Boccadoro M,
Sonneveld P. European Myeloma Network Group review and consensus
statement on primary plasma cell leukemia. Ann Oncol. 2025
Apr;36(4):361-374. doi: 10.1016/j.annonc.2025.01.022. https://doi.org/10.1016/j.annonc.2025.01.022 PMid:39924085
- Bohra
A, Hassan R, Zanwar S, Jevremovic D, Olteanu H, Gonsalves WI, Otteson
G, Horna P, Rajkumar SV, Kumar S. Peripheral Blood Flow Cytometry-based
Definition of Plasma Cell Leukemia. Leukemia. 2025 May 26. doi:
10.1038/s41375-025-02653-z https://doi.org/10.1038/s41375-025-02653-z PMid:40419655
- Bruinink
DH, Kuiper R, van Duin M, Cupedo T, van der Velden V, Hoogenboezem R,
van der Holt B, Berveloo O, Valent ET, Vermeulen M, et al.
Identification of high-risk multiple myeloma with a plasma cell
leukemia-like transcriptomic profile. J Clin Oncol 2022; 40: 3132-3150 https://doi.org/10.1200/JCO.21.01217 PMid:35357885 PMCid:PMC9509081
- Liang
D, Yan Y, Bai S, Xu W, Wang Q, Feng D, Bu Y, Zeng M, Nie X, Feng Y,
Chen X, Xia Z, Liang Y, Jin F, Wang H. Clinical outcome of ≥2%
circulating tumor cells in newly diagnosed multiple myeloma: insights
from a multicenter study. Ann Med. 2025 Dec;57(1):2496796. doi:
10.1080/07853890.2025.2496796 https://doi.org/10.1080/07853890.2025.2496796 PMid:40304724 PMCid:PMC12044910
- Jin
X, Jiang X, Li H, Shen K, Liu S, Chen M, Yang C, Han B, Zhuang J.
Prognostic Implications of Circulating Plasma Cell Percentage in
Multiple Myeloma and Primary Plasma Cell Leukemia Defined by New
Criteria. Acta Haematol. 2025;148(1):48-57. doi: 10.1159/000538658.
Epub 2024 Apr 1 https://doi.org/10.1159/000538658 PMid:38626745 PMCid:PMC11809457
- Jelinek
T, Bezdekova R, Zihala D, Seccikova T, Sithara AA, Pospisilova L,
Sevcikova S, Pothakova P, Stork M, Knecktova Z, et al. More than 2% of
circulating tumor plasma cell leukemia-like multiple myeloma. J Clin
Oncol 2023; 41: 13831392 https://doi.org/10.1200/JCO.22.01226 PMid:36315921 PMCid:PMC9995102
- Deng
J, Qin X, Ma G, Shen X, Sun J, Zhao Y, Zhang Z, Sun Y, Jie G, Su L, Ma
J, Tian W, Yang L, Wang Q, Huang H, Shi M, Ma Y, Gao W, Chen W.
Development of a clinical prognostic model for primary plasma cell
leukemia patients treated with novel agents: a multicenter
retrospective cohort study. Ann Hematol. 2024 July 19
Sep;103(9):3691-3699. doi: 10.1007/s00277-024-05862-1. Epub 2024 https://doi.org/10.1007/s00277-024-05862-1 PMid:39073588
- Cazaubiel
T, Leleu X, Perrot A, Manier S, Buisson L, Maheo S, Do Souto Ferreira
L, Lannes R, Pavageau L, Hulin C, et al. Primary plasma cell leukemias
displaying t(11;14) have specific genomic, transcriptional and clinical
features. Blood 2022; 139 (17): 2666- 2672. https://doi.org/10.1182/blood.2021014968 PMid:35171994
- Gonzalez-Paz
NC, Van Wier S, Santana-Davila R, Ahmann G, Price-Troska T, Henderson
K, Gertz M, Kyle R, Greipp P, et al. Plasma cells from secondary plasma
cell leukemia display different cytogenetics pattern when compared with
primary plasma cell leukemia. Blood 2005; 106: 3267. https://doi.org/10.1182/blood.V106.11.3267.3267
- Tessier
C, LeBlanc R, Roy J, Trudel S, Côté J, Lalancette M, Boudreault JS,
Lemieux-Blanchard É, Kaedbey R, Pavic M. Poor outcome despite modern
treatments: A retrospective study of 99 patients with primary and
secondary plasma cell leukemia. Cancer Med. 2024; 13(17):e70192. doi:
10.1002/cam4.70192. https://doi.org/10.1002/cam4.70192 PMid:39225552 PMCid:PMC11369989
- Manimaran
P, Rai V, Ranka R, Sawhney J. Plasma Cell Leukemia-Clinicopathological
Profile from a Tertiary Care Center in Western India. South Asian J
Cancer. 2023 Jun 9;12(3):280-285. doi: 10.1055/s-0043-57231. https://doi.org/10.1055/s-0043-57231 PMid:38047050 PMCid:PMC10691916
- Valentini
CG, Bozzoli V, Fianchi L, Voso MT, Di Paolantonio G, Criscuolo M, Leone
G, Larocca LM, Pagano L. Primary plasma cell leukemia followed by
testicular plasmacytoma. Int J Hematol. 2011 Feb;93(2):224-227. doi:
10.1007/s12185-010-0745-z. https://doi.org/10.1007/s12185-010-0745-z PMid:21229400
- Ca
Vela-Ojeda J, Ramirez-Alvarado A, Sanchez-Rodriguez AS, Garcia-Chavez
J, Montiel-Cervantes LA. Extraosseous Plasmacytoma Confers Poor
Outcomes in Primary Plasma Cell Leukemia. Arch Med Res. 2025 Mar
24;56(5):103207. doi:10.1016/j.arcmed.2025.103207 https://doi.org/10.1016/j.arcmed.2025.103207 PMid:40132256
- Rasillo
A, Tabernero MD, Sánchez ML, Pérez de Andrés M, Martín Ayuso M,
Hernández J, Moro MJ, Fernández-Calvo J, Sayagués JM, Bortoluci A, San
Miguel JF, Orfao A. Fluorescence in situ hybridization analysis of
aneuploidization patterns in monoclonal gammopathy of undetermined
significance versus multiple myeloma and plasma cell leukemia. Cancer.
2003 Feb 1;97(3):601-9. doi: 10.1002/cncr.11100. https://doi.org/10.1002/cncr.11100 PMid:12548602
- García-Sanz
R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, Fernández-Calvo
J, Sanz MA, Pérez-Simón JA, Rasillo A, Miguel JF. Primary plasma cell
leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic
characteristics. Blood. 1999 Feb 1;93(3):1032-7 https://doi.org/10.1182/blood.V93.3.1032 PMid:9920853
- Evans
LA, Jevremovic D, Nandakumar B, Dispenzieri A, Buadi FK, Dingli D, Lacy
MQ, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Muchtar E,
Warsame R, Kourelis TV, Go R, Russell S, Lust JA, Lin Y, Siddiqui M,
Kyle RA, Gertz MA, Rajkumar SV, Kumar SK, Gonsalves WI. Utilizing
multiparametric flow cytometry in the diagnosis of patients with
primary plasma cell leukemia. Am J Hematol. 2020 Jun;95(6):637-642.
doi: 10.1002/ajh.25773. https://doi.org/10.1002/ajh.25773 PMid:32129510 PMCid:PMC7217733
- Bezdekova
R, Jelinek T, Kralova R, Stork M, Polackova P, Vsianska P, Brozova L,
Jarkovsky J, Almasi M, Boichuk I, Knechtova Z, Penka M, Pour L,
Sevcikova S, Hajek R, Rihova L. Necessity of flow cytometry assessment
of circulating plasma cells and its connection with clinical
characteristics of primary and secondary plasma cell leukaemia. Br J
Haematol. 2021 Oct;195(1):95-107. doi: 10.1111/bjh.17713 https://doi.org/10.1111/bjh.17713 PMid:34500493 PMCid:PMC9292932
- Cazuabiel
T, Buisson L, Maheo S, Do Souto Ferreira L, Lannes R, Perrot A, Hulin
C, Avet- Loiuseau H, Corre J. The genomic and transcriptomic landscape
of plasma cell leukemia. Blood 2020; 136 (suppl.1): 48-49. https://doi.org/10.1182/blood-2020-139340
- Chu
B, Wang Y, Shi L, Sun K, Bao L. Combination of circulating plasma cells
enhances the efficacy of R2-ISS stage system for risk classification of
newly diagnosed multiple myeloma: a single-center real-world study.
Blood 2023; 142 (suppl.1): 4740. https://doi.org/10.1182/blood-2023-181006
- Schinke
C, Boyle EM, Ashby C. Wang Y, Lyzogubov V, Wandell C, Qu P, Hoering A,
Deshpande S, Ryank K, et al. Genomic analysis of primary plasma cell
leukemia reveals complex structural alterations and high-risk
mutational patterns. Blood Cancer J 2020; 10:70 https://doi.org/10.1038/s41408-020-0336-z PMid:32555163 PMCid:PMC7303180
- Cazaubiel
T, Leleu X, Perrot A, Manier S, Buisson L, Maheo S, Do Souto Ferreira
L, Lannes R, Pavageau L, Hulin C, et al. Primary plasma cell leukemias
displaying t(11;14) have specific genomic, transcriptional and clinical
features. Blood 2022; 109: 2666-2672 https://doi.org/10.1182/blood.2021014968 PMid:35171994
- Van
de Donk N, Minnema MC, van der Holt B, Schjesvold F, Wu KL, Broijl A,
Roeloffzen W, Gradisseur A, Pietrantuono G, et al. Treatment of primary
plasma cell leukemia with carfilzomib and lenalidomide-based therapy
(EMN12/HOVON-129): final analysis of a non-randomised, multicentre,
phase 2 study. Lancet Oncol 2023, 24: 1119-1133 https://doi.org/10.1016/S1470-2045(23)00405-9 PMid:37717583
- Papadhimitriou
SI, Terpos E, Liapis K, Pavlidis D, Marinakis T, Kastritis E,
Dimopoulos MA, Tsitsilonis QE, Kostopoulos IV. The cytogenetic profile
of primary and secondary plasma cell leukemia: etiopathogenetic
perspectives, prognostic impact and clinical relevance to newly
diagnosed multiple myeloma with differential circulating clonal plasma
cells. Biomedicines 2022; 10: 209. https://doi.org/10.3390/biomedicines10020209 PMid:35203419 PMCid:PMC8869452
- Skerget
S, Penaherrera D, Chari A, et Al. Comprehensive molecular profiling of
multiple myeloma identifies refined copy number and expression
subtypes. Nat Genet. 2024 Sep;56(9):1878-1889
- Skerget
S, Peneherrera D, Mikhael J, Keats JJ. High-risk gene expression
subtype provides molecular basis for different clinical presentation of
primary and secondary plasma cell leukemia. Blood 2021; 138(suppl.1):
726. https://doi.org/10.1182/blood-2021-148138
- Lee
Y, Lee N, Yoon SS, Yoon SH, Lee DS. Distinct genetic features in
peripheral blood represent the characteristics of circulating plasma
cells in primary plasma cell leukemia. Blood 2023; 142( suppl.1): 3349.
https://doi.org/10.1182/blood-2023-179646
- Rojas
EA, Gutiérrez NC. Genomics of Plasma Cell Leukemia. Cancers (Basel).
2022 Mar 21;14(6):1594. doi: 10.3390/cancers14061594. https://doi.org/10.3390/cancers14061594 PMid:35326746 PMCid:PMC8946729
- D'Agostino
M, Cairns DA, Lahuerta JJ, Wester R, Bertsch U, Waage A, Zamagni E,
Mateos MV, Dall'Olio D, van de Donk NWCJ, Jackson G, Rocchi S,
Salwender H, Bladé Creixenti J, van der Holt B, Castellani G, Bonello
F, Capra A, Mai EK, Dürig J, Gay F, Zweegman S, Cavo M, Kaiser MF,
Goldschmidt H, Hernández Rivas JM, Larocca A, Cook G, San-Miguel JF,
Boccadoro M, Sonneveld P. Second Revision of the International Staging
System (R2-ISS) for Overall Survival in Multiple Myeloma: A European
Myeloma Network (EMN) Report Within the HARMONY Project. J Clin Oncol.
2022 Oct 10;40(29):3406-3418. doi: 10.1200/JCO.21.02614. Epub 2022 May
23. Erratum in: J Clin Oncol. 2022 Dec 1;40(34):4032. doi:
10.1200/JCO.22.02228. https://doi.org/10.1200/JCO.22.02228 PMid:36260864
- Jurczyszyn
A, Olszewska-Szopa M, Vesole DH. The Current State of Knowledge About
Evolution of Multiple Myeloma to Plasma Cell Leukemia. Clin Lymphoma
Myeloma Leuk. 2023 Mar;23(3):188-193. doi: 10.1016/j.clml.2022.12.002. https://doi.org/10.1016/j.clml.2022.12.002 PMid:36593169
- Granell
M, Calvo X, Garcia-Guiñón A, Escoda L, Abella E, Martínez CM, Teixidó
M, Gimenez MT, Senín A, Sanz P, Campoy D, Vicent A, Arenillas L,
Rosiñol L, Sierra J, Bladé J, de Larrea CF; GEMMAC (Grup per l'estudi
del mieloma i l'amiloïdosi de Catalunya). Prognostic impact of
circulating plasma cells in patients with multiple myeloma:
implications for plasma cell leukemia definition. Haematologica. 2017
Jun;102(6):1099-1104. doi: 10.3324/haematol.2016.158303 https://doi.org/10.3324/haematol.2016.158303 PMid:28255016 PMCid:PMC5451342
- Jurczyszyn
A, Radocha J, Davila J, Fiala MA, Gozzetti A, Grząśko N, Robak P, Hus
I, Waszczuk-Gajda A, Guzicka-Kazimierczak R, Atilla E, Mele G, Sawicki
W, Jayabalan DS, Charliński G, Szabo AG, Hajek R, Delforge M, Kopacz A,
Fantl D, Waage A, Avivi I, Rodzaj M, Leleu X, Richez V,
Knopińska-Posłuszny W, Masternak A, Yee AJ, Barchnicka A, Druzd-Sitek
A, Guerrero-Garcia T, Liu J, Vesole DH, Castillo JJ. Prognostic
indicators in primary plasma cell leukaemia: a multicentre
retrospective study of 117 patients. Br J Haematol. 2018
Mar;180(6):831-839. doi: 10.1111/bjh.15092. https://doi.org/10.1111/bjh.15092 PMid:29315478
- Katodritou
E, Terpos E, Delimpasi S, Kotsopoulou M, Michalis E, Vadikolia C,
Kyrtsonis MC, Symeonidis A, Giannakoulas N, Vadikolia C, Michael M,
Kalpadakis C, Gougopoulou T, Prokopiou C, Kaiafa G, Christoulas D,
Gavriatopoulou M, Giannopoulou E, Labropoulou V, Verrou E, Kastritis E,
Konstantinidou P, Anagnostopoulos A, Dimopoulos MA. Real-world data on
prognosis and outcome of primary plasma cell leukemia in the era of
novel agents: a multicenter national study by the Greek Myeloma Study
Group. Blood Cancer J. 2018 Mar 9;8(3):31. doi:
10.1038/s41408-018-0059-6. https://doi.org/10.1038/s41408-018-0059-6 PMid:29523783 PMCid:PMC5849880
- Tian
M, An G, Fu W, Yan W, Li L, Sun C, Li Z, Chen L, Liao A, Gao G, Qin X,
Li M, Li C, Xue H, Gao L, Wang Y, He A, Zhou F, Guo D, Dong Y, Fang Z,
Chu X, Mi J, Fu C, Zeng H, Hou S, Wang X, Wang H, Wei Y, Liang X, Yi X,
Sun Y, Qiu L, Dai Y, Jin F. Development and validation of a prognostic
staging system for primary plasma cell leukemia. J Hematol Oncol. 2025
Jul 15;18(1):72. doi: 10.1186/s13045-025-01723-0 https://doi.org/10.1186/s13045-025-01723-0 PMid:40660267 PMCid:PMC12261572
- Saba
L, Landau KS, Liang H, Fu CL, Chaulagain CP. Real world analysis on the
determinants of survival in primary plasma cell leukemia in the United
States. Leukemia. 2024 Feb;38(2):435-437. doi:
10.1038/s41375-023-02100-x. https://doi.org/10.1038/s41375-023-02100-x PMid:38049508
- Dimopoulos
MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell
leukaemia. Br J Haematol. 1994 Dec;88(4):754-9. doi:
10.1111/j.1365-2141.1994.tb05114.x https://doi.org/10.1111/j.1365-2141.1994.tb05114.x PMid:7819100
- Sica
S, Chiusolo P, Salutari P, Piccirillo N, Laurenti L, Ortu La Barbera E,
Serra FG, Leone G. Long-lasting complete remission in plasma cell
leukemia after aggressive chemotherapy and CD34-selected autologous
peripheral blood progenitor cell transplant: molecular follow-up of
minimal residual disease. Bone Marrow Transplant. 1998 Oct;22(8):823-5.
doi: 10.1038/sj.bmt.1701420. https://doi.org/10.1038/sj.bmt.1701420 PMid:9827984
- Usmani
SZ, Nair B, Qu P, Hansen E, Zhang Q, Petty N, Waheed S, Shaughnessy JD
Jr, Alsayed Y, Heuck CJ, van Rhee F, Milner T, Hoering A, Szymonifka J,
Sexton R, Sawyer J, Singh Z, Crowley J, Barlogie B. Primary plasma cell
leukemia: clinical and laboratory presentation, gene-expression
profiling and clinical outcome with Total Therapy protocols. Leukemia.
2012 Nov;26(11):2398-405. doi: 10.1038/leu.2012.107 https://doi.org/10.1038/leu.2012.107 PMid:22508408 PMCid:PMC3426639
- Pagano
L, Valentini CG, De Stefano V, Venditti A, Visani G, Petrucci MT,
Candoni A, Specchia G, Visco C, Pogliani EM, Ferrara F, Galieni P,
Gozzetti A, Fianchi L, De Muro M, Leone G, Musto P, Pulsoni A; for
GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute
Leukemia Working Party: coordinator Sergio Amadori). Primary plasma
cell leukemia: a retrospective multicenter study of 73 patients. Ann
Oncol. 2011 Jul;22(7):1628-1635. doi: 10.1093/annonc/mdq646. https://doi.org/10.1093/annonc/mdq646 PMid:21252060
- Musto
P, Rossini F, Gay F, et al: Efficacy and safety of bortezomib in
patients with plasma cell leukemia. Cancer 2007; 109:2285-2290. https://doi.org/10.1002/cncr.22700 PMid:17469169
- Raab
MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple
myeloma. Lancet. 2009 Jul 25;374(9686):324-39. doi:
10.1016/S0140-6736(09)60221-X. https://doi.org/10.1016/S0140-6736(09)60221-X PMid:19541364
- D'Arena
G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, Mansueto
G, Villani O, Onofrillo D, Falcone A, Specchia G, Semenzato G, Di Renzo
N, Mastrullo L, Venditti A, Ferrara F, Palumbo A, Pagano L, Musto P.
Frontline chemotherapy with bortezomib-containing combinations improves
response rate and survival in primary plasma cell leukemia: a
retrospective study from GIMEMA Multiple Myeloma Working Party. Ann
Oncol. 2012 Jun;23(6):1499-502. doi: 10.1093/annonc/mdr480. https://doi.org/10.1093/annonc/mdr480 PMid:22039089
- Musto
P, Simeon V, Todoerti K, Neri A. Primary Plasma Cell Leukemia: Identity
Card 2016. Curr Treat Options Oncol. 2016 Apr;17(4):19. doi:
10.1007/s11864-016-0392-6 https://doi.org/10.1007/s11864-016-0392-6 PMid:26995215
- Royer
B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C, Arnulf B, Macro
M, Cailleres S, Brion A, Brechignac S, Belhadj K, Chretien ML,
Wetterwald M, Chaleteix C, Tiab M, Leleu X, Frenzel L, Garderet L,
Choquet S, Fuzibet JG, Dauriac C, Forneker LM, Benboubker L, Facon T,
Moreau P, Avet-Loiseau H, Marolleau JP. Bortezomib, Doxorubicin,
Cyclophosphamide, Dexamethasone Induction Followed by Stem Cell
Transplantation for Primary Plasma Cell Leukemia: A Prospective Phase
II Study of the Intergroupe Francophone du Myélome. J Clin Oncol. 2016
Jun 20;34(18):2125-32. doi: 10.1200/JCO.2015.63.1929. https://doi.org/10.1200/JCO.2015.63.1929 PMid:27114594
- Pagano
L, Maraglino AME, Fianchi L, Criscuolo M, Rossi E, Za T, Chiusolo P,
Bonanni M, Dragonetti G, Bacigalupo A, Sica S, De Stefano V. Treatment
of primary plasma cell leukemia with high doses of cyclophosphamide,
bortezomib, and dexamethasone followed by double autologous HSCT. Ann
Hematol. 2020 Jan;99(1):207-209. doi: 10.1007/s00277-019-03885-7. https://doi.org/10.1007/s00277-019-03885-7 PMid:31832752
- Houbaida
Y., Del Giudice M.L., Galimberti S., Buda G. How first-line therapy is
changing in transplant-eligible multiple myeloma patients. Mediterr J
Hematol Infect Dis 2025, 17(1): e2025026, DOI: http://dx.doi.org/10.4084/MJHID.2025.026 https://doi.org/10.4084/MJHID.2025.026 PMid:40084095 PMCid:PMC11906123
- Mina
R, Joseph NS, Kaufman JL, Gupta VA, Heffner LT, Hofmeister CC, Boise
LH, Dhodapkar MV, Gleason C, Nooka AK, Lonial S. Survival outcomes of
patients with primary plasma cell leukemia (pPCL) treated with novel
agents. Cancer 2019; 125: 416-423. https://doi.org/10.1002/cncr.31718 PMid:30332496
- Singh
S, Peshin S, Wertheim BC, Larsen A, Chineke I, Sborov DW, Green D,
Liedtke M, Okoniewski M, Wazir M, Nadeem O, Schachter LG, DeGraaff D,
Vardell VA, Coffey DG, Gowin K. Outcomes and treatment patterns in
primary and secondary plasma cell leukemia: insights from a large US
cohort study. Haematologica. 2025 Sep 1;110(9):2129-2138. doi:
10.3324/haematol.2024.287158. https://doi.org/10.3324/haematol.2024.287158 PMCid:PMC12399943
- Parrondo
RD, Moustafa MA, Reeder C, Sher T, Roy V, Muchtar E, Warsame R, Alegria
V, Gonsalves W, Dingli D, Hayman S, Kapoor P, Chanan-Khan AA, Ailawadhi
S. Efficacy of Daratumumab-Based Regimens for the Treatment of Plasma
Cell Leukemia. Clin Lymphoma Myeloma Leuk. 2021 May;21(5):355-360. doi:
10.1016/j.clml.2021.01.002. https://doi.org/10.1016/j.clml.2021.01.002 PMid:33563579
- Cotte
C, Hartley-Brown M. Plasma cell leukemia: Retrospective review of cases
at Monter Cancer Center/Northwell Health Cancer Institute, 2014-2019.
Curr Probl Cancer. 2022 Jun;46(3):100831. doi:
10.1016/j.currproblcancer.2021.100831. https://doi.org/10.1016/j.currproblcancer.2021.100831 PMid:35091270
- Li
AY, Kamangar F, Holtzman NG,Rapoport AP, Kocoglu MH, Atanackovic D,
Badros AZ. A Clinical Perspective on Plasma Cell Leukemia: A
Single-Center Experience. Cancers (Basel). 2024 Jun 5;16(11):2149. doi:
10.3390/cancers16112149. https://doi.org/10.3390/cancers16112149 PMid:38893268 PMCid:PMC11172213
- Katodritou
E, Kastritis E, Dalampira D, Delimpasi S, Spanoudakis E, Labropoulou V,
Ntanasis-Stathopoulos I, Gkioka AI, Giannakoulas N, Kanellias N,
Papadopoulou T, Sevastoudi A, Michalis E, Papathanasiou M, Kotsopoulou
M, Sioni A, Triantafyllou T, Daiou A, Papadatou M, Kyrtsonis MC, Pouli
A, Kostopoulos I, Verrou E, Dimopoulos MA, Terpos E. Improved survival
of patients with primary plasma cell leukemia with VRd or
daratumumab-based quadruplets: A multicenter study by the Greek myeloma
study group. Am J Hematol. 2023 May;98(5):730-738. doi:
10.1002/ajh.26891. https://doi.org/10.1002/ajh.26891 PMid:36869876
- Shalaby
K, Azad F, Parker S, Wang C, Yu H, Attwood K, Hillengass J. Clinical
Characteristics and Survival Outcomes of Patients With Primary and
Secondary Plasma Cell Leukemia According to the 2021 Definition: A
Single Center Retrospective Study. Clin Lymphoma Myeloma Leuk. 2025
Jan;25(1):67-75 https://doi.org/10.1016/j.clml.2024.10.014 PMid:39578203
- Rees
MJ, D'Agostino M, Leypoldt LB, Kumar S, Weisel KC, Gay F. Navigating
High-Risk and Ultrahigh-Risk Multiple Myeloma: Challenges and Emerging
Strategies. Am Soc Clin Oncol Educ Book. 2024 Jun;44(3):e433520. doi:
10.1200/EDBK_433520. https://doi.org/10.1200/EDBK_433520 PMid:38772002
- Usmani
SZ, Hoering A, Ailawadhi S, Sexton R, Lipe B, Hita SF, Valent J,
Rosenzweig M,, Zonder JA, Dhodapkar M, Callander N, Zimmerman T,
Voorhees PM, Durie B, Rajkumar SV, Richardson PG, Orlowski RZ; SWOG1211
Trial Investigators. Bortezomib, lenalidomide, and dexamethasone with
or without elotuzumab in patients with untreated, high-risk multiple
myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial.
Lancet Haematol. 2021 Jan;8(1):e45-e54. doi:
10.1016/S2352-3026(20)30354-9. https://doi.org/10.1016/S2352-3026(20)30354-9 PMid:33357482
- Kaiser
MF, Hall A, Walker K, Sherborne A, De Tute RM, Newnham N, Roberts S,
Ingleson E, Bowles K, Garg M, Lokare A, Messiou C, Houlston RS, Jackson
G, Cook G, Pratt G, Owen RG, Drayson MT, Brown SR, Jenner MW.
Daratumumab, Cyclophosphamide, Bortezomib, Lenalidomide, and
Dexamethasone as Induction and Extended Consolidation Improves Outcome
in Ultra-High-Risk Multiple Myeloma. J Clin Oncol. 2023 Aug
10;41(23):3945-3955. doi: 10.1200/JCO.22.02567. https://doi.org/10.1200/JCO.22.02567 PMid:37315268
- Leypoldt
LB, Tichy D, Besemer B, Hänel M, Raab MS, Mann C, Munder M, Reinhardt
HC, Nogai A, Görner M, Ko YD, de Wit M, Salwender H, Scheid C, Graeven
U, Peceny R, Staib P, Dieing A, Einsele H, Jauch A, Hundemer M, Zago M,
Požek E, Benner A, Bokemeyer C, Goldschmidt H, Weisel KC.) Isatuximab,
Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of
High-Risk Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2024 Jan
1;42(1):26-37. doi: 10.1200/JCO.23.01696. https://doi.org/10.1200/JCO.23.01696 PMid:37753960 PMCid:PMC10730063
- Dhakal
B, Patel S, Girnius S, Bachegowda L, Fraser R, Davila O, Kanate AS,
Assal A, Hanbali A, Bashey A, Pawarode A, Freytes CO, Lee C, Vesole D,
Cornell RF, Hildebrandt GC, Murthy HS, Lazarus HM, Cerny J, Yared JA,
Schriber J, Berdeja J, Stockerl-Goldstein K, Meehan K, Holmberg L, Solh
M, Diaz MA, Kharfan-Dabaja MA, Farhadfar N, Bashir Q, Munker R, Olsson
RF, Gale RP, Bayer RL, Seo S, Chhabra S, Hashmi S, Badawy SM, Nishihori
T, Gonsalves W, Nieto Y, Efebera Y, Kumar S, Shah N, Qazilbash M, Hari
P, D'Souza A. Hematopoietic cell transplantation utilization and
outcomes for primary plasma cell leukemia in the current era. Leukemia.
2020 Dec;34(12):3338-3347. doi: 10.1038/s41375-020-0830-0. Epub 2020
Apr 20. Erratum in: Leukemia. 2021 Jun;35(6):1828. doi:
10.1038/s41375-021-01233-1. Erratum in: Leukemia. 2021 Jul;35(7):2141.
doi: 10.1038/s41375-021-01304-3. https://doi.org/10.1038/s41375-021-01304-3 PMid:34091601
- Lawless
S, Iacobelli S, Knelange NS, Chevallier P, Blaise D, Milpied N, Foà R,
Cornelissen JJ, Lioure B, Benjamin R, Poiré X, Minnema MC, Collin M,
Lenhoff S, Snowden JA, Santarone S, Wilson KMO, Trigo F, Dreger P,
Böhmer LH, Putter H, Garderet L, Kröger N, Yaukoub-Agha I, Schönland S,
Morris C. Comparison of autologous and allogeneic hematopoietic cell
transplantation strategies in patients with primary plasma cell
leukemia, with dynamic prediction modeling. Haematologica. 2023 Apr
1;108(4):1105-1114. doi: 10.3324/haematol.2021.280568. https://doi.org/10.3324/haematol.2021.280568 PMid:35770529 PMCid:PMC10071135
- Maegaki
M, Takahashi T, Suzuki K, Minakata D, Terao T, Satake A, Akira H, Oyake
T, Kanda Y, Aotsuka N, Tsukada N, Tabayashi T, Kobayashi H, Nawa Y,
Ichinohe T, Ohbiki M, Atsuta Y, Kawamura K. Comparison of Autologous
and Allogeneic Hematopoietic Stem Cell Transplantation for Primary
Plasma Cell Leukemia in Japan. Transplant Cell Ther. 2025 Sep
8:S2666-6367(25)01402-2. doi: 10.1016/j.jtct.2025.08.026. https://doi.org/10.1016/j.jtct.2025.08.026 PMid:40930225
- Rees
MJ, Kumar S. High-risk multiple myeloma: Redefining genetic, clinical,
and functional high-risk disease in the era of molecular medicine and
immunotherapy. Am J Hematol. 2024 Aug;99(8):1560-1575. doi:
10.1002/ajh.27327 https://doi.org/10.1002/ajh.27327 PMid:38613829
- Szita
VR, Mikala G, Kozma A, Fábián J, Hardi A, Alizadeh H, Rajnics P, Rejtő
L, Szendrei T, Váróczy L, Nagy Z, Illés Á, Vályi-Nagy I, Masszi T,
Varga G. Targeted Venetoclax Therapy in t(11;14) Multiple Myeloma: Real
World Data From Seven Hungarian Centers. Pathol Oncol Res. 2022 Feb
28;28:1610276. doi: 10.3389/pore.2022.1610276 https://doi.org/10.3389/pore.2022.1610276 PMid:35295611 PMCid:PMC8918485
- Usmani
SZ, Hoering A, Ailawadhi S, Sexton R, Lipe B, Hita SF, Valent J,
Rosenzweig MAlvanidis G, Kotsos D, Frouzaki C, Fola A, Hatjiharissi E.
The potential role of BCL-2 inhibition in amyloidosis and plasma cell
leukemia. Front Oncol. 2025 Mar 21;15:1549891. doi:
10.3389/fonc.2025.1549891 https://doi.org/10.3389/fonc.2025.1549891 PMid:40190562 PMCid:PMC11968654
- Gonsalves
WI, Buadi FK, Kumar SK. Combination therapy incorporating Bcl-2
inhibition with Venetoclax for the treatment of refractory primary
plasma cell leukemia with t (11;14). Eur J Haematol. (2018) 100:215-7.
doi: 10.1111/ejh.2018.100.issue-2 https://doi.org/10.1111/ejh.2018.100.issue-2
- Nalghranyan
S, Singh AP, Schinke C. The combination of venetoclax, daratumumab and
dexamethasone for the treatment of refractory primary plasma cell
leukemia. Am J Hematol. (2020) 95:E34-5. doi: 10.1002/ajh.25676 https://doi.org/10.1002/ajh.25676 PMid:31709578
- Valliani
S, Ali M, Mahmoo O, Hinduja S, Chen CK, Damon L, et al. Efficacy of
venetoclax and dexamethasone in refractory IgM primary plasma cell
leukemia with t(11;14) and TP53 mutation: A case report and literature
review. Case Rep Hematol. (2020) 2020:8823877. doi:
10.1155/2020/882387735. https://doi.org/10.1155/2020/8823877 PMid:33425404 PMCid:PMC7781713
- Tang
ASO, Ahmad Asnawi AW, Koh AZY, Chong SL, Liew PK, Selvaratnam V, et al.
Plasma cell leukemia with successful upfront venetoclax in combination
with allogeneic transplantation. Am J Case Rep. (2023) 24:e938868. doi:
10.12659/AJCR.938868 https://doi.org/10.12659/AJCR.938868 PMid:36882990 PMCid:PMC10009647
- Charalampous
C, Doucette K, Chappell A, Vesole DH. Venetoclax-based induction
therapy for primary plasma cell leukemia with high BCL-2 expression.
Leuk Lymphoma. (2024) 65(12):1901-4. doi: 10.1080/10428194.2024.2381647
https://doi.org/10.1080/10428194.2024.2381647 PMid:39088749
- Vo
K, Guan T, Banerjee R, Lo M, Young R, Shah N. Complete response
following treatment of plasma cell leukemia with venetoclax and
dexamethasone: A case report. J Oncol Pharm Pract. (2022) 28:1244-8.
doi: 10.1177/10781552221074269 https://doi.org/10.1177/10781552221074269 PMid:35084252
- Roy
T, An JB, Doucette K, Chappell AM, Vesole DH. Venetoclax in upfront
induction therapy for primary plasma cell leukemia with t(11;14) or
BCL2 expression. Leuk Lymphoma. (2022) 63:759-61. doi:
10.1080/10428194.2021.2010065 https://doi.org/10.1080/10428194.2021.2010065 PMid:35076333
- Yang
Y, Fu LJ, Chen CM, HuMW. Venetoclax in combination with chidamide and
dexamethasone in relapsed/refractory primary plasma cell leukemia
without t(11;14): A case report. World J Clin Cases. (2021) 9:1175-83.
doi: 10.12998/wjcc.v9.i5.117540.76. https://doi.org/10.12998/wjcc.v9.i5.1175 PMid:33644182 PMCid:PMC7896656
- Kupsh
A, Arnall J, Voorhees P. A successful case of venetoclax-based therapy
in relapsed/refractory secondary plasma cell leukemia. J Oncol Pharm
Pract. (2020)26:1274-8. doi: 10.1177/1078155219895072 https://doi.org/10.1177/1078155219895072 PMid:31865846
- Glavey
SV, Flanagan L, Bleach R, Kelly C, Quinn J, Nı́ Chonghaile T, et al.
Secondary plasma cell leukaemia treated with single agent venetoclax.
Br J Haematol. (2020) 190:e242-5. doi: 10.1111/bjh.v190.4 https://doi.org/10.1111/bjh.v190.4
- Elsabah
H, Ghasoub R, El Omri H, Benkhadra M, Cherif H, Taha RY. Venetoclax in
the treatment of secondary plasma cell leukemia with translocation
t(11;14): a case report and literature review. Front Oncol. (2024)
14:1390747. doi: 10.3389/fonc.2024.1390747 https://doi.org/10.3389/fonc.2024.1390747 PMid:39050574 PMCid:PMC11266074
- Morgan
HT, Derman BA, Ma H, Kumar SK. Changing lanes: extending CAR T-cell
therapy to high-risk plasma cell dyscrasias. Front Immunol. 2025 Apr
8;16:1558275. doi: 10.3389/fimmu.2025.1558275 https://doi.org/10.3389/fimmu.2025.1558275 PMid:40264764 PMCid:PMC12011880
- Fortuna
GMG, Sidana S, Hovanky V, Khouri J, Dima D, Kocoglu MH, et al.
Idecabtagene vicleucel (ide-cel) chimeric antigen receptor T-cell for
plasma cell leukemia (PCL): A multicenter experience. Transplant Cell
Ther Off Publ Am Soc Transplant Cell Ther. (2024) 30:S381-S2. doi:
10.1016/j.jtct.2023.12.534 121. https://doi.org/10.1016/j.jtct.2023.12.534
- Guo
Y, Hu K, Ke X, Yang F, Ma L, Xu T, et al. PB2101: A prospective
investigation into the timing and status of BCMA-CART in secondary
plasma cell leukemia. HemaSphere. (2023) 7:e640168c. doi:
10.1097/01.HS9.0000975188.64016.8c https://doi.org/10.1097/01.HS9.0000975188.64016.8c PMCid:PMC10428716
- Li
C, Cao W, Que Y, Wang Q, Xiao Y, Gu C, et al. A phase I study of
anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma
and plasma cell leukemia. Clin Transl Med. (2021) 11:e346. doi:
10.1002/ctm2.v11.333. https://doi.org/10.1002/ctm2.346 PMid:33784005 PMCid:PMC7943908
- Deng
J, Lin Y, Zhao D, Tong C, Chang AH, Chen W, et al. Case report: Plasma
cell leukemia secondary to multiple myeloma successfully treated with
anti-BCMA CAR-T cell therapy. Front Oncol. (2022) 12:901266. doi:
10.3389/fonc.2022.90126684. https://doi.org/10.3389/fonc.2022.901266 PMid:36212423 PMCid:PMC9533140
- Li
C, Wang Q, Zhu H, Mao X, Wang Y, Zhang Y, et al. T cells expressing
anti-BCMA chimeric antigen receptors for plasma cell Malignancies.
Blood. (2018) 132:1013-. doi: 10.1182/blood-2018-99-116898 https://doi.org/10.1182/blood-2018-99-116898
- Li
C, Cao W, Que Y, Wang Q, Xiao Y, Gu C, Wang D, Wang J, Jiang L, Xu H,
Xu J, Zhou X, Hong Z, Wang N, Huang L, Zhang S, Chen L, Mao X, Xiao M,
Zhang W, Meng L, Cao Y, Zhang T, Li J, Zhou J. A phase I study of
anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma
and plasma cell leukemia. Clin Transl Med. 2021 Mar;11(3):e346. doi:
10.1002/ctm2.346. 17(1): e2025045, DOI:
http://dx.doi.org/10.4084/MJHID.2025.045 https://doi.org/10.4084/MJHID.2025.045 PMid:40375902 PMCid:PMC12081044
- Han
W, Wang S, Zhang M, Fu S, Wu W, Zhao H, Wong KW, Yip SF, Cui J, Chang
AH, Wei G, Huang H, Hu Y. Sequential intrathoracic injection and
intravenous infusion of BCMA CAR-T cells in a patient with
relapsed/refractory primary plasma cell leukemia. Int J Hematol. 2025
Sep 10. doi: 10.1007/s12185-025-04064-3 https://doi.org/10.1007/s12185-025-04064-3 PMid:40931245
- Kasap
C, Izgutdina A, Patiño-Escobar B, Kang AS, Chilakapati N, Akagi N,
Manoj A, Johnson H, Rashid T, Werner J, Barpanda A, Geng H, Lin YT,
Rampersaud S, Gil-Alós D, Sobh A, Dupéré-Richer D, Aleman A, Wicaksono
G, Kelii KMK, Dalal R, Ramos E, Vijayanarayanan A, Lakhani K,
Salangsang F, Phojanakong P, Camara Serrano JA, Zakraoui O, Tariq I,
Chari A, Chung A, Kumar AD, Martin TG 3rd, Wolf J, Wong SW, Steri V,
Shanmugam M, Boise LH, Kortemme T, Parekh S, Stieglitz E, Licht JD,
Karlon WJ, Barwick BG, Wiita AP. Targeting high-risk multiple myeloma
genotypes with optimized anti-CD70 CAR T cells. Blood. 2025 Aug
14;146(7):819-833. doi: 10.1182/blood.2024025536 https://doi.org/10.1182/blood.2024025536 PMid:40359480
- Puppi
M., Sacchetti I., Mancuso K., Tacchetti P., Pantani L., Rizzello I.,
Iezza M., Talarico M., Manzato E., Masci S., Restuccia R., Barbato S.,
Armuzzi S., Taurisano B., Vigliotta I., Zamagni E. Bispecific
antibodies and CART in multiple myeloma: appropriate selection of
patients and sequencing. Mediterr J Hematol Infect Dis 2025 https://doi.org/10.4084/MJHID.2025.045 PMid:40375902 PMCid:PMC12081044
- Bernardi, C.; Beauverd, Y.; Tran, T.A.; Maulini, M.; Mappoura, M.; Morin, S.; Simonetta, F.; Cairoli, A.; Auner, H.W.; Samii, K.; et al. Anti-BCMA and GPRC5D bispecific antibodies in relapsed/refractory primary plasma cell leukemia: A case report. Front. Immunol. 2024, 15, 1495233. https://doi.org/10.3389/fimmu.2024.1495233 PMid:39676854 PMCid:PMC11638231